Cortical Implications of Advancing Age and Disease Duration in Parkinson's Disease Patients with Postural Instability and Gait Dysfunction
- PMID: 27164041
- PMCID: PMC5826542
- DOI: 10.3233/JPD-150753
Cortical Implications of Advancing Age and Disease Duration in Parkinson's Disease Patients with Postural Instability and Gait Dysfunction
Abstract
Background: Parkinson's Disease patients with predominant gait dysfunction appear to have reduced cortical thickness compared to other motor phenotypes. The extent to which advancing age or disease duration impact the pattern of these distinctions is unclear.
Objective: We examine if PD patients with predominant signs of postural instability and gait dysfunction are distinguished by distinct patterns of cerebral atrophy, and how these differences are influenced by age and disease duration.
Methods: The Unified Parkinson's Disease Rating Score (UPDRS) was administered to 196 PD patients (age = 61.4±8.9yrs) in the Off and On dopamine state. All completed a structural T1-weighted brain MRI. We defined 3 motor phenotypes: tremor dominant, akinetic-rigid, and postural instability with gait disorder. General linear modeling quantified cortical thickness in relation to disease duration, and motor improvement after dopaminergic therapy. Cortical thickness and subcortical volumes were compared between the three motor subtypes, after controlling for disease duration and age.
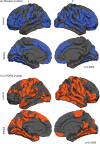
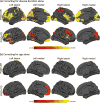
Results: We identified 177/196 patients who met criteria for a motor subtype. When corrected for disease duration, postural-instability patients had marked cortical thinning of the bilateral frontal-temporal and posterior cortical regions (cuneus/precuneus). After regressing for age, reduced frontal thickness was evident in patients with gait dysfunction. Widespread cortical thinning was associated with increasing disease duration and reduced motor improvement to dopaminergic therapy.
Conclusions: Results emphasize that the profile of motor signs, especially prominent gait manifestations, relate to cortical thinning in distinct regions. Unique patterns of atrophy appear to be driven by advancing pathology related to age and disease duration.
Keywords: MRI; PIGD; Parkinson’s disease; gait disorders; motor phenotypes; tremor dominant.
Conflict of interest statement
The authors have no relevant conflicts of interest to report and no other relevant financial relationships to disclose other than listed.
Figures
Similar articles
-
Cortical complexity alterations in motor subtypes of Parkinson's disease: A surface-based morphometry analysis of fractal dimension.Eur J Neurosci. 2024 Dec;60(12):7249-7262. doi: 10.1111/ejn.16612. Epub 2024 Dec 3. Eur J Neurosci. 2024. PMID: 39627178
-
Different patterns of spontaneous brain activity between tremor-dominant and postural instability/gait difficulty subtypes of Parkinson's disease: a resting-state fMRI study.CNS Neurosci Ther. 2015 Oct;21(10):855-66. doi: 10.1111/cns.12464. CNS Neurosci Ther. 2015. PMID: 26387576 Free PMC article.
-
Regional homogeneity alterations differentiate between tremor dominant and postural instability gait difficulty subtypes of Parkinson's disease.J Neural Transm (Vienna). 2016 Mar;123(3):219-29. doi: 10.1007/s00702-015-1490-5. Epub 2015 Dec 14. J Neural Transm (Vienna). 2016. PMID: 26666253
-
Nonmotor features of Parkinson's disease subtypes.Mov Disord. 2016 Aug;31(8):1095-102. doi: 10.1002/mds.26510. Epub 2016 Feb 10. Mov Disord. 2016. PMID: 26861861 Review.
-
Why we should study gait initiation in Parkinson's disease.Neurophysiol Clin. 2014 Jan;44(1):69-76. doi: 10.1016/j.neucli.2013.10.127. Epub 2013 Oct 30. Neurophysiol Clin. 2014. PMID: 24502907 Review.
Cited by
-
Comprehensive subtyping of Parkinson's disease patients with similarity fusion: a case study with BioFIND data.NPJ Parkinsons Dis. 2021 Sep 17;7(1):83. doi: 10.1038/s41531-021-00228-0. NPJ Parkinsons Dis. 2021. PMID: 34535682 Free PMC article.
-
Altered connectivity between frontal cortex and supplementary motor area in various types of Parkinson's disease.Am J Transl Res. 2024 Jun 15;16(6):2423-2434. doi: 10.62347/GTVB7800. eCollection 2024. Am J Transl Res. 2024. PMID: 39006296 Free PMC article.
-
Cerebral cortical thinning in Parkinson's disease depends on the age of onset.PLoS One. 2023 Feb 21;18(2):e0281987. doi: 10.1371/journal.pone.0281987. eCollection 2023. PLoS One. 2023. PMID: 36809440 Free PMC article.
-
Parkinson's disease multimodal imaging: F-DOPA PET, neuromelanin-sensitive and quantitative iron-sensitive MRI.NPJ Parkinsons Dis. 2021 Jul 8;7(1):57. doi: 10.1038/s41531-021-00199-2. NPJ Parkinsons Dis. 2021. PMID: 34238927 Free PMC article.
-
Cerebral cortical thickness and cognitive decline in Parkinson's disease.Cereb Cortex Commun. 2023 Jan 14;4(1):tgac044. doi: 10.1093/texcom/tgac044. eCollection 2023. Cereb Cortex Commun. 2023. PMID: 36660417 Free PMC article.
References
-
- Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I, Stern M, Tanner C, Weiner W Parkinson Study Group Variable expression of Parkinson’s disease: A base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40:1529–1534. - PubMed
-
- Rajput AH, Voll A, Rajput ML, Robinson CA, Rajput A. Course in Parkinson disease subtypes: A 39-year clinicopathologic study. Neurology. 2009;73:206–212. - PubMed
-
- Schiess MC, Zheng H, Soukup VM, Bonnen JG, Nauta HJ. Parkinson’s disease subtypes: Clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism Relat Disord. 2000;6:69–76. - PubMed
-
- Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord. 2006;21:1123–1130. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

