Spatial Geometries of Self-Assembled Chitohexaose Monolayers Regulate Myoblast Fusion
- PMID: 27164094
- PMCID: PMC4881512
- DOI: 10.3390/ijms17050686
Spatial Geometries of Self-Assembled Chitohexaose Monolayers Regulate Myoblast Fusion
Abstract
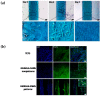
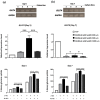
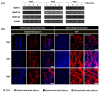
Myoblast fusion into functionally-distinct myotubes to form in vitro skeletal muscle constructs under differentiation serum-free conditions still remains a challenge. Herein, we report that our microtopographical carbohydrate substrates composed of bioactive hexa-N-acetyl-d-glucosamine (GlcNAc6) modulated the efficiency of myoblast fusion without requiring horse serum or any differentiation medium during cell culture. Promotion of the differentiation of dissociated mononucleated skeletal myoblasts (C2C12; a mouse myoblast cell line) into robust myotubes was found only on GlcNAc6 micropatterns, whereas the myoblasts on control, non-patterned GlcNAc6 substrates or GlcNAc6-free patterns exhibited an undifferentiated form. We also examined the possible role of GlcNAc6 micropatterns with various widths in the behavior of C2C12 cells in early and late stages of myogenesis through mRNA expression of myosin heavy chain (MyHC) isoforms. The spontaneous contraction of myotubes was investigated via the regulation of glucose transporter type 4 (GLUT4), which is involved in stimulating glucose uptake during cellular contraction. Narrow patterns demonstrated enhanced glucose uptake rate and generated a fast-twitch muscle fiber type, whereas the slow-twitch muscle fiber type was dominant on wider patterns. Our findings indicated that GlcNAc6-mediated integrin interactions are responsible for guiding myoblast fusion forward along with myotube formation.
Keywords: GLUT4; cell fusion; chitohexaose; glucose uptake; micropattern; myoblast; myosin heavy chain; skeletal muscle.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

