Expression of Evc2 in craniofacial tissues and craniofacial bone defects in Evc2 knockout mouse
- PMID: 27164562
- PMCID: PMC4903910
- DOI: 10.1016/j.archoralbio.2016.05.002
Expression of Evc2 in craniofacial tissues and craniofacial bone defects in Evc2 knockout mouse
Abstract
Objective: Our objectives were to determine the expression of EVC2 in craniofacial tissues and investigate the effect of Evc2 deficiency on craniofacial bones using Evc2 knockout (KO) mouse model.
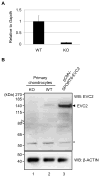
Design: Evc2 KO mice were generated by introducing a premature stop codon followed by the Internal Ribosomal Entry Site fused to β-galactosidase (LacZ). Samples from wild-type (WT), heterozygous (Het) and homozygous Evc2 KO mice were prepared. LacZ staining and immunohistochemistry (IHC) with anti-β-galactosidase, anti-EVC2 and anti-SOX9 antibodies were performed. The craniofacial bones were stained with alcian blue and alizarin red.
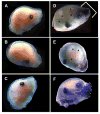
Results: The LacZ activity in KO was mainly observed in the anterior parts of viscerocranium. The Evc2-expressing cells were identified in many cartilageous regions by IHC with anti-β-galactosidase antibody in KO and Het embryos. The endogenous EVC2 protein was observed in these areas in WT embryos. Double labeling with anti-SOX9 antibody showed that these cells were mainly chondrocytes. At adult stages, the expression of EVC2 was found in chondrocytes of nasal bones and spheno-occipital synchondrosis, and osteocytes and endothelial-like cells of the premaxilla and mandible. The skeletal double staining demonstrated that craniofacial bones, where the expression of EVC2 was observed, in KO had the morphological defects as compared to WT.
Conclusion: To our knowledge, our study was the first to identify the types of Evc2-expressing cells in craniofacial tissues. Consistent with the expression pattern, abnormal craniofacial bone morphology was found in the Evc2 KO mice, suggesting that EVC2 may be important during craniofacial growth and development.
Keywords: Craniofacial bone; Development; EVC2; Ellis-van Creveld syndrome; Expression; Knockout (KO) mouse.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing interests: The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures




Similar articles
-
Ellis Van Creveld2 is Required for Postnatal Craniofacial Bone Development.Anat Rec (Hoboken). 2016 Aug;299(8):1110-20. doi: 10.1002/ar.23353. Epub 2016 May 2. Anat Rec (Hoboken). 2016. PMID: 27090777 Free PMC article.
-
The Role of Ellis-Van Creveld 2(EVC2) in Mice During Cranial Bone Development.Anat Rec (Hoboken). 2018 Jan;301(1):46-55. doi: 10.1002/ar.23692. Epub 2017 Oct 6. Anat Rec (Hoboken). 2018. PMID: 28950429 Free PMC article.
-
Identification of Compound Heterozygous EVC2 Gene Variants in Two Mexican Families with Ellis-van Creveld Syndrome.Genes (Basel). 2023 Apr 9;14(4):887. doi: 10.3390/genes14040887. Genes (Basel). 2023. PMID: 37107645 Free PMC article.
-
Role of primary cilia and Hedgehog signaling in craniofacial features of Ellis-van Creveld syndrome.Am J Med Genet C Semin Med Genet. 2022 Mar;190(1):36-46. doi: 10.1002/ajmg.c.31969. Epub 2022 Apr 8. Am J Med Genet C Semin Med Genet. 2022. PMID: 35393766 Review.
-
Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands.Am J Med Genet C Semin Med Genet. 2009 Nov 15;151C(4):341-51. doi: 10.1002/ajmg.c.30226. Am J Med Genet C Semin Med Genet. 2009. PMID: 19876929 Review.
Cited by
-
A Ciliary Protein EVC2/LIMBIN Plays a Critical Role in the Skull Base for Mid-Facial Development.Front Physiol. 2018 Oct 25;9:1484. doi: 10.3389/fphys.2018.01484. eCollection 2018. Front Physiol. 2018. PMID: 30410447 Free PMC article.
-
Whole exome sequencing identified sixty-five coding mutations in four neuroblastoma tumors.Sci Rep. 2017 Dec 19;7(1):17787. doi: 10.1038/s41598-017-17162-y. Sci Rep. 2017. PMID: 29259192 Free PMC article.
-
Molecular and Cellular Pathogenesis of Ellis-van Creveld Syndrome: Lessons from Targeted and Natural Mutations in Animal Models.J Dev Biol. 2020 Oct 9;8(4):25. doi: 10.3390/jdb8040025. J Dev Biol. 2020. PMID: 33050204 Free PMC article. Review.
-
Loss of Function of Evc2 in Dental Mesenchyme Leads to Hypomorphic Enamel.J Dent Res. 2017 Apr;96(4):421-429. doi: 10.1177/0022034516683674. Epub 2017 Jan 12. J Dent Res. 2017. PMID: 28081373 Free PMC article.
-
Cranial Base Synchondrosis: Chondrocytes at the Hub.Int J Mol Sci. 2022 Jul 15;23(14):7817. doi: 10.3390/ijms23147817. Int J Mol Sci. 2022. PMID: 35887171 Free PMC article. Review.
References
-
- Polymeropoulos MH, Ide SE, Wright M, Goodship J, Weissenbach J, Pyeritz RE, et al. The gene for the Ellis-van Creveld syndrome is located on chromosome 4p16. Genomics. 1996;35(1):1–5. - PubMed
-
- Ruiz-Perez VL, Ide SE, Strom TM, Lorenz B, Wilson D, Woods K, et al. Mutations in a new gene in Ellis-van Creveld syndrome and Weyers acrodental dysostosis. Nat Genet. 2000;24(3):283–286. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

