Comparative interactomics analysis of different ALS-associated proteins identifies converging molecular pathways
- PMID: 27164932
- PMCID: PMC4947123
- DOI: 10.1007/s00401-016-1575-8
Comparative interactomics analysis of different ALS-associated proteins identifies converging molecular pathways
Abstract
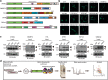
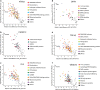
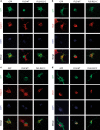
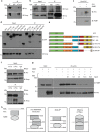
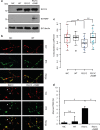
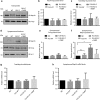
Amyotrophic lateral sclerosis (ALS) is a devastating neurological disease with no effective treatment available. An increasing number of genetic causes of ALS are being identified, but how these genetic defects lead to motor neuron degeneration and to which extent they affect common cellular pathways remains incompletely understood. To address these questions, we performed an interactomic analysis to identify binding partners of wild-type (WT) and ALS-associated mutant versions of ATXN2, C9orf72, FUS, OPTN, TDP-43 and UBQLN2 in neuronal cells. This analysis identified several known but also many novel binding partners of these proteins. Interactomes of WT and mutant ALS proteins were very similar except for OPTN and UBQLN2, in which mutations caused loss or gain of protein interactions. Several of the identified interactomes showed a high degree of overlap: shared binding partners of ATXN2, FUS and TDP-43 had roles in RNA metabolism; OPTN- and UBQLN2-interacting proteins were related to protein degradation and protein transport, and C9orf72 interactors function in mitochondria. To confirm that this overlap is important for ALS pathogenesis, we studied fragile X mental retardation protein (FMRP), one of the common interactors of ATXN2, FUS and TDP-43, in more detail in in vitro and in vivo model systems for FUS ALS. FMRP localized to mutant FUS-containing aggregates in spinal motor neurons and bound endogenous FUS in a direct and RNA-sensitive manner. Furthermore, defects in synaptic FMRP mRNA target expression, neuromuscular junction integrity, and motor behavior caused by mutant FUS in zebrafish embryos, could be rescued by exogenous FMRP expression. Together, these results show that interactomics analysis can provide crucial insight into ALS disease mechanisms and they link FMRP to motor neuron dysfunction caused by FUS mutations.
Keywords: Amyotrophic lateral sclerosis; C9orf72; FMRP; FUS; Motor neuron; TDP-43.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

