Characterization of the Nanog 5'-flanking Region in Bovine
- PMID: 27165025
- PMCID: PMC5003962
- DOI: 10.5713/ajas.16.0032
Characterization of the Nanog 5'-flanking Region in Bovine
Abstract
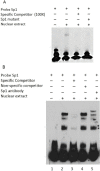
Bovine embryonic stem cells have potential for use in research, such as transgenic cattle generation and the study of developmental gene regulation. The Nanog may play a critical role in maintenance of the undifferentiated state of embryonic stem cells in the bovine, as in murine and human. Nevertheless, efforts to study the bovine Nanog for pluripotency-maintaining factors have been insufficient. In this study, in order to understand the mechanisms of transcriptional regulation of the bovine Nanog, the 5'-flanking region of the Nanog was isolated from ear cells of Hanwoo. Results of transient transfection using a luciferase reporter gene under the control of serially deleted 5'-flanking sequences revealed that the -134 to -19 region contained the positive regulatory sequences for the transcription of the bovine Nanog. Results from mutagenesis studies demonstrated that the Sp1-binding site that is located in the proximal promoter region plays an important role in transcriptional activity of the bovine Nanog promoter. The electrophoretic mobility shift assay with the Sp1 specific antibody confirmed the specific binding of Sp1 transcription factor to this site. In addition, significant inhibition of Nanog promoter activity by the Sp1 mutant was observed in murine embryonic stem cells. Furthermore, chromatin-immunoprecipitation assay with the Sp1 specific antibody confirmed the specific binding of Sp1 transcription factor to this site. These results suggest that Sp1 is an essential regulatory factor for bovine Nanog transcriptional activity.
Keywords: Bovine; Embryonic Stem Cells; Nanog; Sp1; Transcription Factors.
Figures



References
-
- Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. - PubMed
-
- Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol. 2002;195:27–38. - PubMed
-
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. - PubMed
-
- Chan KK, Zhang J, Chia NY, Chan YS, Sim HS, Tan KS, Oh SK, Ng HH, Choo AB. KLF4 and PBX1 directly regulate NANOG expression in human embryonic stem cells. Stem Cells. 2009;27:2114–2125. - PubMed
-
- Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. Differences between human and mouse embryonic stem cells. Dev Biol. 2004;269:360–380. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

