Proliferation and Migration of Peripheral Retinal Pigment Epithelial Cells Are Associated with the Upregulation of Wingless-Related Integration and Bone Morphogenetic Protein Signaling in Dark Agouti Rats
- PMID: 27165129
- PMCID: PMC5588433
- DOI: 10.1159/000446480
Proliferation and Migration of Peripheral Retinal Pigment Epithelial Cells Are Associated with the Upregulation of Wingless-Related Integration and Bone Morphogenetic Protein Signaling in Dark Agouti Rats
Abstract
Objective: The aim of this study was to investigate the possible migration of proliferating peripheral retinal pigment epithelial (RPE) cells and their association with differential gene expressions.
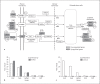
Materials and methods: The RPE layer was obtained from the inner aspect of the eyeball of dark agouti rats (12-13 weeks old) and was mounted on glass slides. The peripheral RPE cell proliferation was evaluated using bromodeoxyuridine immunohistochemistry (n = 10). The cell migration was examined using the Dil tracer technique (n = 40) at the end of weeks 6, 10, 14 and 18. Affymetrix microarray analysis was used to investigate differential gene expressions in peripheral and central RPE cells, which was authenticated by RT-PCR using 4 RPE-specific genes (n = 10).
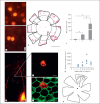
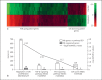
Results: In this study, peripheral RPE cells divided and appeared in clusters, but equatorial and central RPE cells rarely divided. The peripheral RPE cells migrated to the central RPE region in a time-dependent manner up to the end of week 14, but not later. The microarray analysis showed the expression of 9,645 out of a total of 35,220 genes studied. Among the 9,645 genes, 573 were differentially expressed (438 were upregulated and 135 were downregulated) in peripheral RPE cells as compared to central RPE cells. Of these 573 genes, 56 were associated with signaling pathways related to the regulation of cell proliferation, including Pax6, TGFβ, BMP and Wnt, and 404 were associated with pathways of cell migration.
Conclusions: In this study, peripheral RPE cells divided and migrated to the central region. This process was associated with differential gene expressions in these cells.
© 2016 S. Karger AG, Basel.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

