Reversal of pentylenetetrazole-altered swimming and neural activity-regulated gene expression in zebrafish larvae by valproic acid and valerian extract
- PMID: 27165438
- PMCID: PMC4908174
- DOI: 10.1007/s00213-016-4304-z
Reversal of pentylenetetrazole-altered swimming and neural activity-regulated gene expression in zebrafish larvae by valproic acid and valerian extract
Abstract
Rationale: Ethnopharmacology has documented hundreds of psychoactive plants awaiting exploitation for drug discovery. A robust and inexpensive in vivo system allowing systematic screening would be critical to exploiting this knowledge.
Objective: The objective of this study was to establish a cheap and accurate screening method which can be used for testing psychoactive efficacy of complex mixtures of unknown composition, like plant crude extracts.
Methods: We used automated recording of zebrafish larval swimming behavior during light vs. dark periods which we reproducibly altered with an anxiogenic compound, pentylenetetrazole (PTZ). First, we reversed this PTZ-altered swimming by co-treatment with a well-defined synthetic anxiolytic drug, valproic acid (VPA). Next, we aimed at reversing it by adding crude root extracts of Valeriana officinalis (Val) from which VPA was originally derived. Finally, we assessed how expression of neural activity-regulated genes (c-fos, npas4a, and bdnf) known to be upregulated by PTZ treatment was affected in the presence of Val.
Results: Both VPA and Val significantly reversed the PTZ-altered swimming behaviors. Noticeably, Val at higher doses was affecting swimming independently of the presence of PTZ. A strong regulation of all three neural-activity genes was observed in Val-treated larvae which fully supported the behavioral results.
Conclusions: We demonstrated in a combined behavioral-molecular approach the strong psychoactivity of a natural extract of unknown composition made from V. officinalis. Our results highlight the efficacy and sensitivity of such an approach, therefore offering a novel in vivo screening system amenable to high-throughput testing of promising ethnobotanical candidates.
Keywords: Crude extracts; Ethnobotany; In vivo high-throughput psychoactivity screening; Psychoactive plants; Valerian; Zebrafish larva behavior; bdnf; c-fos; npas4a.
Figures

 ) and PTZ7.5-treated (
) and PTZ7.5-treated ( ) larvae reported minute by minute for the entire 80 min of recording. After an adaptation period to dark (gray box), larvae were submitted to four successive 10-min alternating light (L1–L4: white zones) and dark periods (D1–D4: gray zones). b Distances traveled in all successive post-transitions (=first minutes after a light switch) by untreated (
) larvae reported minute by minute for the entire 80 min of recording. After an adaptation period to dark (gray box), larvae were submitted to four successive 10-min alternating light (L1–L4: white zones) and dark periods (D1–D4: gray zones). b Distances traveled in all successive post-transitions (=first minutes after a light switch) by untreated ( ) and PTZ7.5-treated larvae (
) and PTZ7.5-treated larvae ( ). c Distances traveled in cumulative post-transitions in light (L 1–4, white boxes) and in dark (D 1–4, grey boxes). d Distances traveled during all successive non-transitions (= remaining 9 min of each light or dark periods). e Distances traveled in compiled non-transitions. f Inner distances traveled in swim speed S2 (0.2 cm/s < S2 < 2 cm/s) in compiled post-transitions. g Inner distances traveled in swim speed S3 (>2 cm/s) in compiled post-transitions. h Inner distances traveled in all successive post-transitions in swim speed S2 (
). c Distances traveled in cumulative post-transitions in light (L 1–4, white boxes) and in dark (D 1–4, grey boxes). d Distances traveled during all successive non-transitions (= remaining 9 min of each light or dark periods). e Distances traveled in compiled non-transitions. f Inner distances traveled in swim speed S2 (0.2 cm/s < S2 < 2 cm/s) in compiled post-transitions. g Inner distances traveled in swim speed S3 (>2 cm/s) in compiled post-transitions. h Inner distances traveled in all successive post-transitions in swim speed S2 ( ) and S3 (
) and S3 ( ). Error bars representing SEM. *p values <0.001 (detailed values are presented in Supplementary Table 1)
). Error bars representing SEM. *p values <0.001 (detailed values are presented in Supplementary Table 1)
 ) and VPA-alone-treated (
) and VPA-alone-treated ( ) larvae in top panels, and by PTZ7.5-alone-treated (
) larvae in top panels, and by PTZ7.5-alone-treated ( ) and VPA + PTZ7.5-co-treated (
) and VPA + PTZ7.5-co-treated ( ) larvae in bottom panels. Larvae were treated with increasing VPA concentrations: 0.5 mM (VPA0.5, left column), 1 mM (VPA1, second column), 2 mM (VPA2, third column), and 3 mM (VPA3, fourth column). b Distances traveled during all successive post-transitions by untreated (
) larvae in bottom panels. Larvae were treated with increasing VPA concentrations: 0.5 mM (VPA0.5, left column), 1 mM (VPA1, second column), 2 mM (VPA2, third column), and 3 mM (VPA3, fourth column). b Distances traveled during all successive post-transitions by untreated ( ), PTZ7.5-alone-treated (
), PTZ7.5-alone-treated ( ), VPA2 alone (
), VPA2 alone ( ), and PTZ7.5 + VPA2-co-treated larvae (
), and PTZ7.5 + VPA2-co-treated larvae ( ). c Distances traveled during cumulative post-transitions. d Distances traveled during all successive non-transitions (= remaining 9 min of each light or dark periods). e Distances traveled during compiled non-transitions. f Inner distances traveled in swim speed S2 (0.2 cm/s < S2 < 2 cm/s) during compiled post-transitions. g Inner distances traveled in swim speed S3 (>2 cm/s) during compiled post-transitions. h Inner distances traveled during all compiled post-transitions in swim speed S2 (
). c Distances traveled during cumulative post-transitions. d Distances traveled during all successive non-transitions (= remaining 9 min of each light or dark periods). e Distances traveled during compiled non-transitions. f Inner distances traveled in swim speed S2 (0.2 cm/s < S2 < 2 cm/s) during compiled post-transitions. g Inner distances traveled in swim speed S3 (>2 cm/s) during compiled post-transitions. h Inner distances traveled during all compiled post-transitions in swim speed S2 ( ) and S3 (
) and S3 ( ) by untreated (U), PTZ7.5-alone-treated (P), VPA2-alone-treated (V), and VPA2 + PTZ7.5-co-treated (VP) larvae. Error bars represent SEM. Symbols for significant p values were omitted for clarity but are discussed in the “Results” section and detailed in Supplementary Tables 2, 3, 4, and 5
) by untreated (U), PTZ7.5-alone-treated (P), VPA2-alone-treated (V), and VPA2 + PTZ7.5-co-treated (VP) larvae. Error bars represent SEM. Symbols for significant p values were omitted for clarity but are discussed in the “Results” section and detailed in Supplementary Tables 2, 3, 4, and 5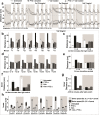
 ) and Val-alone-treated (
) and Val-alone-treated ( ) larvae in top panels, and by PTZ7.5-alone-treated (
) larvae in top panels, and by PTZ7.5-alone-treated ( ) and Val + PTZ7.5-co-treated (
) and Val + PTZ7.5-co-treated ( ) larvae in bottom panels. Larvae were treated with increasing Val concentrations: 1 mg/ml (Val1, left column), 2.5 mg/ml (Val2.5, second column), 5 mg/ml (Val5, third column), and 7 mg/ml (Val7, fourth column). b Distances traveled during all successive post-transitions by untreated (
) larvae in bottom panels. Larvae were treated with increasing Val concentrations: 1 mg/ml (Val1, left column), 2.5 mg/ml (Val2.5, second column), 5 mg/ml (Val5, third column), and 7 mg/ml (Val7, fourth column). b Distances traveled during all successive post-transitions by untreated ( ), PTZ7.5-alone-treated (
), PTZ7.5-alone-treated ( ), Val5 alone (
), Val5 alone ( ), and Val5 + PTZ7.5-co-treated larvae (
), and Val5 + PTZ7.5-co-treated larvae ( ). c Distances traveled during compiled post-transitions. d Distances traveled during all compiled non-transitions. e Distances traveled during compiled non-transitions. f Inner distances traveled in swim speed S2 (0.20 cm/s < S2 < 2 cm/s) during compiled post-transitions. g Inner distances traveled in swim speed S3 (>2 cm/s) during cumulative post-transitions. h Inner distances traveled during all successive post-transitions in swim speed S2 (
). c Distances traveled during compiled post-transitions. d Distances traveled during all compiled non-transitions. e Distances traveled during compiled non-transitions. f Inner distances traveled in swim speed S2 (0.20 cm/s < S2 < 2 cm/s) during compiled post-transitions. g Inner distances traveled in swim speed S3 (>2 cm/s) during cumulative post-transitions. h Inner distances traveled during all successive post-transitions in swim speed S2 ( ) and S3 (
) and S3 ( ) by untreated (U), PTZ7.5-alone-treated (P), Val5-alone-treated (V), and Val5 + PTZ7.5-co-treated (VP) larvae. Error bars represent SEM. Symbols for significant p values were omitted for clarity but are discussed in the “Results” section and detailed in Supplementary Tables 6, 7, 8, and 9
) by untreated (U), PTZ7.5-alone-treated (P), Val5-alone-treated (V), and Val5 + PTZ7.5-co-treated (VP) larvae. Error bars represent SEM. Symbols for significant p values were omitted for clarity but are discussed in the “Results” section and detailed in Supplementary Tables 6, 7, 8, and 9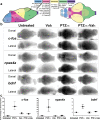
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

