Interactions among filamentous fungi Aspergillus niger, Fusarium verticillioides and Clonostachys rosea: fungal biomass, diversity of secreted metabolites and fumonisin production
- PMID: 27165654
- PMCID: PMC4862089
- DOI: 10.1186/s12866-016-0698-3
Interactions among filamentous fungi Aspergillus niger, Fusarium verticillioides and Clonostachys rosea: fungal biomass, diversity of secreted metabolites and fumonisin production
Abstract
Background: Interactions among fungi colonizing dead organic matter involve exploitation competition and interference competition. Major mechanism of interference competition is antibiosis caused by secreted secondary metabolites. The effect of competition on secondary metabolite production by fungi is however poorly understood. Fungal biomass was rarely monitored in interaction studies; it is not known whether dominance in pairwise interactions follows congruent patterns.
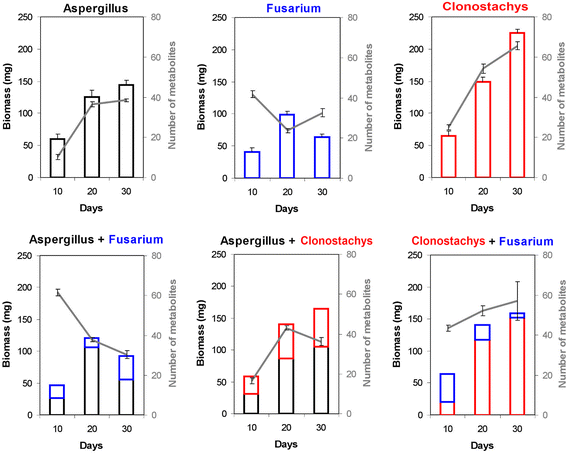
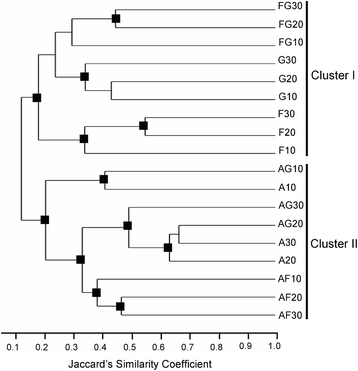
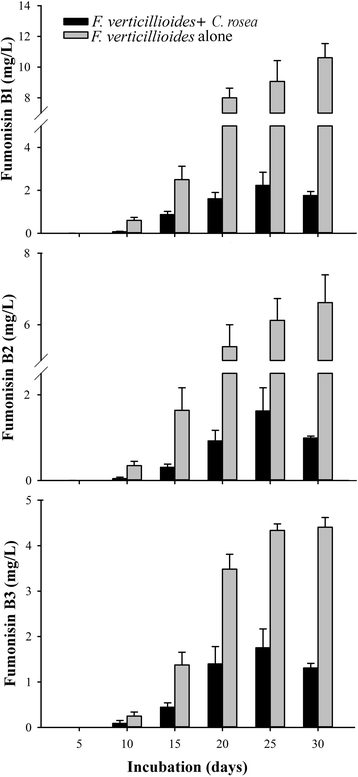
Results: Pairwise interactions of three fungal species with different life styles were studied. The saprophyte Aspergillus niger (A.n.), the plant pathogen Fusarium verticillioides (F.v.), and the mycoparasite Clonostachys rosea (C.r.) were grown in single and dual cultures in minimal medium with asparagine as nitrogen source. Competitive fitness shifted with time: in dual C.r./F.v. cultures after 10 d F.v. grew well while C.r. was suppressed; after 20 d C.r. recovered while F.v. became suppressed; and after 30 d most F.v. was destroyed. At certain time points fungal competitive fitness exhibited a rock-paper-scissors pattern: F.v. > A.n., A.n. > C.r., and C.r. > F.v. Most metabolites secreted to the medium at early stages in single and dual cultures were not found at later times. Many metabolites occurring in supernatants of single cultures were suppressed in dual cultures and many new metabolites not occurring in single cultures were found in dual cultures. A. niger showed the greatest ability to suppress the accumulation of metabolites produced by the other fungi. A. niger was also the species with the largest capacity of transforming metabolites produced by other fungi. Fumonisin production by F. verticillioides was suppressed in co-cultures with C. rosea but fumonisin B1 was not degraded by C. rosea nor did it affect the growth of C. rosea up to a concentration of 160 μg/ml.
Conclusions: Competitive fitness in pairwise interactions among fungi is incongruent, indicating that species-specific factors and/or effects are involved. Many metabolites secreted by fungi are catabolized by their producers at later growth stages. Diversity of metabolites accumulating in the medium is stimulated by fungus/fungus interactions. C. rosea suppresses the synthesis of fumonisins by F. verticillioides but does not degrade fumonisins.
Keywords: Fumonisin; Interference competition; Metabolic diversity; Metabolic profiling.
Figures

References
-
- Wicklow DT. Interference competition and the organization of fungal communities. In: Wicklow DT, Carroll GC, editors. The fungal community, its organization and role in the ecosystem. 2. New York: Marcel Dekker; 1992. p. 931.
-
- Sirenko L, Malyarevskaya A, Birger T, Kirpenko Y. Ecological metabolites. In: Krasnov EV, editor. Vzaimodeistne Vodoi Zhiuym Veshchestuom.Volume 2. Moscow: Izdania; 1979. pp. 48–56.
-
- Karlovsky P. Secondary Metabolites in Soil Ecology. In: Karlovsky P, editor. Secondary Metabolites in Soil Ecology, Soil Biology. Berlin Heidelberg: Springer; 2008. pp. 1–19.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

