The clinical performance of the M4 decision support model to triage women with a pregnancy of unknown location as at low or high risk of complications
- PMID: 27165655
- PMCID: PMC4901883
- DOI: 10.1093/humrep/dew105
The clinical performance of the M4 decision support model to triage women with a pregnancy of unknown location as at low or high risk of complications
Abstract
Study question: What are the adverse outcomes associated with using the M4 model in everyday clinical practice for women with pregnancy of unknown location (PUL)?
Summary answer: There were 17/835 (2.0%) adverse events and no serious adverse events associated with the performance of the M4 model in clinical practice.
What is known already: The M4 model has previously been shown to stratify women classified as a PUL as at low or high risk of complications with a good level of test performance. The triage performance of the M4 model is better than single measurements of serum progesterone or the hCG ratio (serum hCG at 48 h/hCG at presentation).
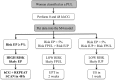
Study design, size, duration: A prospective multi-centre cohort study of 1022 women with a PUL carried out between August 2012 and December 2013 across 2 university teaching hospitals and 1 district general hospital.
Participants/materials, setting, methods: All women presenting with a PUL to the early pregnancy units of the three hospitals were recruited. The final outcome for PUL was either a failed PUL (FPUL), intrauterine pregnancy (IUP) or ectopic pregnancy (EP) (including persistent PUL (PPUL)), with EP and PPUL considered high-risk PUL. Their hCG results at 0 and 48 h were entered into the M4 model algorithm. If the risk of EP was ≥5%, the PUL was predicted to be high-risk and the participant was asked to re-attend 48 h later for a repeat hCG and transvaginal ultrasound scan by a senior clinician. If the PUL was classified as 'low risk, likely failed PUL', the participant was asked to perform a urinary pregnancy test 2 weeks later. If the PUL was classified as 'low risk, likely intrauterine', the participant was scheduled for a repeat scan in 1 week. Deviations from the management protocol were recorded as either an 'unscheduled visit (participant reason)', 'unscheduled visit (clinician reason)' or 'differences in timing (blood test/ultrasound)'. Adverse events were assessed using definitions outlined in the UK Good Clinical Practice Guidelines' document.
Main results and the role of chance: A total of 835 (82%) women classified as a PUL were managed according to the M4 model (9 met the exclusion criteria, 69 were lost to follow-up, 109 had no hCG result at 48 h). Of these, 443 (53%) had a final outcome of FPUL, 298 (36%) an IUP and 94 (11%) an EP. The M4 model predicted 70% (585/835) PUL as low risk, of which 568 (97%) were confirmed as FPUL or IUP. Of the 17 EP and PPUL misclassified as low risk, 5 had expectant management, 7 medical management with methotrexate and 5 surgical intervention.Nineteen PUL had an unscheduled visit (participant reason), 38 PUL had an unscheduled visit (clinician reason) and 68 PUL had deviations from protocol due to a difference in timing (blood test/ultrasound).Adverse events were reported in 26 PUL and 1 participant had a serious adverse event. A total of 17/26 (65%) adverse events were misclassifications of a high risk PUL as low risk by the M4 model, while 5/26 (19%) adverse events were related to incorrect clinical decisions. Four of the 26 adverse events (15%) were secondary to unscheduled admissions for pain/bleeding. The serious adverse event was due to an incorrect clinical decision.
Limitations, reasons for caution: A limitation of the study was that 69/1022 (7%) of PUL were lost to follow-up. A 48 h hCG level was missing for 109/1022 (11%) participants.
Wider implications of the findings: The low number of adverse events (2.0%) suggests that expectant management of PUL using the M4 prediction model is safe. The model is an effective way of triaging women with a PUL as being at high- and low-risk of complications and rationalizing follow-up. The multi-centre design of the study is more likely to make the performance of the M4 model generalizable in other populations.
Study funding/competing interests: None.
Trial registration number: Not applicable.
Keywords: adverse events; decision support techniques; ectopic pregnancy; miscarriage; pregnancy of unknown location; triage; ultrasonography.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


Similar articles
-
Triaging women with pregnancy of unknown location using two-step protocol including M6 model: clinical implementation study.Ultrasound Obstet Gynecol. 2020 Jan;55(1):105-114. doi: 10.1002/uog.20420. Ultrasound Obstet Gynecol. 2020. PMID: 31385381
-
Triaging pregnancies of unknown location: the performance of protocols based on single serum progesterone or repeated serum hCG levels.Hum Reprod. 2014 May;29(5):938-45. doi: 10.1093/humrep/deu045. Epub 2014 Mar 14. Hum Reprod. 2014. PMID: 24634251
-
Classification of pregnancies of unknown location according to four different hCG-based protocols.Hum Reprod. 2016 Oct;31(10):2203-11. doi: 10.1093/humrep/dew202. Epub 2016 Aug 31. Hum Reprod. 2016. PMID: 27580995
-
Diagnostic protocols for the management of pregnancy of unknown location: a systematic review and meta-analysis.BJOG. 2019 Jan;126(2):190-198. doi: 10.1111/1471-0528.15442. Epub 2018 Sep 20. BJOG. 2019. PMID: 30129999
-
Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location.Hum Reprod Update. 2014 Mar-Apr;20(2):250-61. doi: 10.1093/humupd/dmt047. Epub 2013 Oct 6. Hum Reprod Update. 2014. PMID: 24101604 Review.
Cited by
-
Clinical decision support systems for maternity care: a systematic review and meta-analysis.EClinicalMedicine. 2024 Sep 5;76:102822. doi: 10.1016/j.eclinm.2024.102822. eCollection 2024 Oct. EClinicalMedicine. 2024. PMID: 39296586 Free PMC article.
-
Pregnancy of unknown location: external validation of the hCG-based M6NP and M4 prediction models in an emergency gynaecology unit.BMJ Open. 2022 Nov 29;12(11):e058454. doi: 10.1136/bmjopen-2021-058454. BMJ Open. 2022. PMID: 36446455 Free PMC article.
-
Managing Pregnancies of Unknown Location With the M4 Prediction Model or the NICE Algorithm: A Randomised Controlled Trial With Cross-Sectional Diagnostic Accuracy Data.BJOG. 2025 May;132(6):742-751. doi: 10.1111/1471-0528.18079. Epub 2025 Jan 22. BJOG. 2025. PMID: 39840550 Free PMC article. Clinical Trial.
-
The Development of an Ultrasound-Based Scoring System for the Prediction of Interstitial Pregnancy.J Clin Med. 2025 Jun 14;14(12):4238. doi: 10.3390/jcm14124238. J Clin Med. 2025. PMID: 40565983 Free PMC article.
-
Triaging Women with Pregnancy of Unknown Location: Evaluation of Protocols Based on Single Serum Progesterone, Serum hCG Ratios, and Model M4.J Reprod Infertil. 2022 Apr-Jun;23(2):107-113. doi: 10.18502/jri.v23i2.8995. J Reprod Infertil. 2022. PMID: 36043136 Free PMC article.
References
-
- Banerjee S, Aslam N, Zosmer N, Woelfer B, Jurkovic D. The expectant management of women with early pregnancy of unknown location. Ultrasound Obstet Gynecol 1999;14:231–236. - PubMed
-
- Banerjee S, Aslam N, Woelfer B, Lawrence A, Elson J, Jurkovic D. Expectant management of early pregnancies of unknown location: a prospective evaluation of methods to predict spontaneous resolution of pregnancy. BJOG 2001;108:158–163. - PubMed
-
- Bignardi T, Condous G, Alhamdan D, Kirk E, Van Calster B, Van Huffel S, Timmerman D, Bourne T. The hCG ratio can predict the ultimate viability of the intrauterine pregnancies of uncertain viability in the pregnancy of unknown location population. Hum Reprod 2008;23:1964–1967. - PubMed
-
- Bottomley C, Van Belle V, Mukri F, Kirk E, Van Huffel S, Timmerman D, Bourne T. The optimal timing of an ultrasound scan to assess the location and viability of an early pregnancy. Hum Reprod 2009;24:1811–1817. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

