Caloric restriction blocks neuropathology and motor deficits in Machado-Joseph disease mouse models through SIRT1 pathway
- PMID: 27165717
- PMCID: PMC4865854
- DOI: 10.1038/ncomms11445
Caloric restriction blocks neuropathology and motor deficits in Machado-Joseph disease mouse models through SIRT1 pathway
Abstract
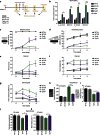
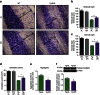
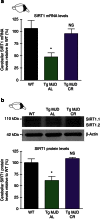
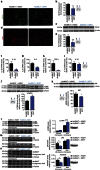
Machado-Joseph disease (MJD) is a neurodegenerative disorder characterized by an abnormal expansion of the CAG triplet in the ATXN3 gene, translating into a polyglutamine tract within the ataxin-3 protein. The available treatments only ameliorate symptomatology and do not block disease progression. In this study we find that caloric restriction dramatically rescues the motor incoordination, imbalance and the associated neuropathology in transgenic MJD mice. We further show that caloric restriction rescues SIRT1 levels in transgenic MJD mice, whereas silencing SIRT1 is sufficient to prevent the beneficial effects on MJD pathology. In addition, the re-establishment of SIRT1 levels in MJD mouse model, through the gene delivery approach, significantly ameliorates neuropathology, reducing neuroinflammation and activating autophagy. Furthermore, the pharmacological activation of SIRT1 with resveratrol significantly reduces motor incoordination of MJD mice. The pharmacological SIRT1 activation could provide important benefits to treat MJD patients.
Figures


Similar articles
-
Silencing mutant ataxin-3 rescues motor deficits and neuropathology in Machado-Joseph disease transgenic mice.PLoS One. 2013;8(1):e52396. doi: 10.1371/journal.pone.0052396. Epub 2013 Jan 22. PLoS One. 2013. PMID: 23349684 Free PMC article.
-
The autophagy-enhancing drug carbamazepine improves neuropathology and motor impairment in mouse models of Machado-Joseph disease.Neuropathol Appl Neurobiol. 2022 Feb;48(1):e12763. doi: 10.1111/nan.12763. Epub 2021 Oct 13. Neuropathol Appl Neurobiol. 2022. PMID: 34432315
-
Neuropeptide Y mitigates neuropathology and motor deficits in mouse models of Machado-Joseph disease.Hum Mol Genet. 2015 Oct 1;24(19):5451-63. doi: 10.1093/hmg/ddv271. Epub 2015 Jul 27. Hum Mol Genet. 2015. PMID: 26220979
-
Molecular Mechanisms and Cellular Pathways Implicated in Machado-Joseph Disease Pathogenesis.Adv Exp Med Biol. 2018;1049:349-367. doi: 10.1007/978-3-319-71779-1_18. Adv Exp Med Biol. 2018. PMID: 29427113 Review.
-
The Neuropathology of Spinocerebellar Ataxia Type 3/Machado-Joseph Disease.Adv Exp Med Biol. 2018;1049:233-241. doi: 10.1007/978-3-319-71779-1_11. Adv Exp Med Biol. 2018. PMID: 29427106 Review.
Cited by
-
FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation.Hum Mol Genet. 2021 May 31;30(11):996-1005. doi: 10.1093/hmg/ddab095. Hum Mol Genet. 2021. PMID: 33822053 Free PMC article.
-
Neurodegenerative diseases: model organisms, pathology and autophagy.J Genet. 2018 Jul;97(3):679-701. J Genet. 2018. PMID: 30027903 Review.
-
A Variant in Genes of the NPY System as Modifier Factor of Machado-Joseph Disease in the Chinese Population.Front Aging Neurosci. 2022 Feb 3;14:822657. doi: 10.3389/fnagi.2022.822657. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35185528 Free PMC article.
-
Identifying Therapeutic Targets for Spinocerebellar Ataxia Type 3/Machado-Joseph Disease through Integration of Pathological Biomarkers and Therapeutic Strategies.Int J Mol Sci. 2020 Apr 26;21(9):3063. doi: 10.3390/ijms21093063. Int J Mol Sci. 2020. PMID: 32357546 Free PMC article. Review.
-
Gene expression changes in cerebellum induced by dietary restriction.Front Mol Neurosci. 2023 May 24;16:1185665. doi: 10.3389/fnmol.2023.1185665. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37293544 Free PMC article.
References
-
- Haberhausen G., Damian M. S., Leweke F. & Muller U. Spinocerebellar ataxia, type 3 (SCA3) is genetically identical to Machado-Joseph disease (MJD). J. Neurol. Sci. 132, 71–75 (1995). - PubMed
-
- Kawaguchi Y. et al.. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat. Genet. 8, 221–228 (1994). - PubMed
-
- Coutinho P. & Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands. a new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology 28, 703–709 (1978). - PubMed
-
- Sequeiros J. et al.. Genetic linkage studies of Machado-Joseph disease with chromosome 14q STRPs in 16 Portuguese-Azorean kindreds. Genomics 21, 645–648 (1994). - PubMed
-
- Durr A. et al.. Spinocerebellar ataxia 3 and Machado-Joseph disease: clinical, molecular, and neuropathological features. Ann. Neurol. 39, 490–499 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

