The Toxoplasma gondii Rhoptry Kinome Is Essential for Chronic Infection
- PMID: 27165797
- PMCID: PMC4959664
- DOI: 10.1128/mBio.00193-16
The Toxoplasma gondii Rhoptry Kinome Is Essential for Chronic Infection
Abstract
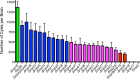
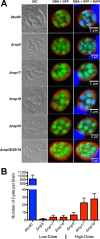
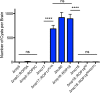
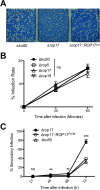
Ingestion of the obligate intracellular protozoan parasite Toxoplasma gondii causes an acute infection that leads to chronic infection of the host. To facilitate the acute phase of the infection, T. gondii manipulates the host response by secreting rhoptry organelle proteins (ROPs) into host cells during its invasion. A few key ROP proteins with signatures of kinases or pseudokinases (ROPKs) act as virulence factors that enhance parasite survival against host gamma interferon-stimulated innate immunity. However, the roles of these and other ROPK proteins in establishing chronic infection have not been tested. Here, we deleted 26 ROPK gene loci encoding 31 unique ROPK proteins of type II T. gondii and show that numerous ROPK proteins influence the development of chronic infection. Cyst burdens were increased in the Δrop16 knockout strain or moderately reduced in 11 ROPK knockout strains. In contrast, deletion of ROP5, ROP17, ROP18, ROP35, or ROP38/29/19 (ROP38, ROP29, and ROP19) severely reduced cyst burdens. Δrop5 and Δrop18 knockout strains were less resistant to host immunity-related GTPases (IRGs) and exhibited >100-fold-reduced virulence. ROP18 kinase activity and association with the parasitophorous vacuole membrane were necessary for resistance to host IRGs. The Δrop17 strain exhibited a >12-fold defect in virulence; however, virulence was not affected in the Δrop35 or Δrop38/29/19 strain. Resistance to host IRGs was not affected in the Δrop17, Δrop35, or Δrop38/29/19 strain. Collectively, these findings provide the first definitive evidence that the type II T. gondii ROPK proteome functions as virulence factors and facilitates additional mechanisms of host manipulation that are essential for chronic infection and transmission of T. gondii
Importance: Reactivation of chronic Toxoplasma gondii infection in individuals with weakened immune systems causes severe toxoplasmosis. Existing treatments for toxoplasmosis are complicated by adverse reactions to chemotherapy. Understanding key parasite molecules required for chronic infection provides new insights into potential mechanisms that can interrupt parasite survival or persistence in the host. This study reveals that key secreted rhoptry molecules are used by the parasite to establish chronic infection of the host. Certain rhoptry proteins were found to be critical virulence factors that resist innate immunity, while other rhoptry proteins were found to influence chronic infection without affecting virulence. This study reveals that rhoptry proteins utilize multiple mechanisms of host manipulation to establish chronic infection of the host. Targeted disruption of parasite rhoptry proteins involved in these biological processes opens new avenues to interfere with chronic infection with the goal to either eliminate chronic infection or to prevent recrudescent infections.
Copyright © 2016 Fox et al.
Figures

Comment in
-
Functional Analysis of the Rhoptry Kinome during Chronic Toxoplasma gondii Infection.mBio. 2016 Jun 14;7(3):e00842-16. doi: 10.1128/mBio.00842-16. mBio. 2016. PMID: 27302762 Free PMC article.
Similar articles
-
Toxoplasma gondii superinfection and virulence during secondary infection correlate with the exact ROP5/ROP18 allelic combination.mBio. 2015 Feb 24;6(2):e02280. doi: 10.1128/mBio.02280-14. mBio. 2015. PMID: 25714710 Free PMC article.
-
Secretion of Rhoptry and Dense Granule Effector Proteins by Nonreplicating Toxoplasma gondii Uracil Auxotrophs Controls the Development of Antitumor Immunity.PLoS Genet. 2016 Jul 22;12(7):e1006189. doi: 10.1371/journal.pgen.1006189. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27447180 Free PMC article.
-
Toxoplasma gondii Parasitophorous Vacuole Membrane-Associated Dense Granule Proteins Orchestrate Chronic Infection and GRA12 Underpins Resistance to Host Gamma Interferon.mBio. 2019 Jul 2;10(4):e00589-19. doi: 10.1128/mBio.00589-19. mBio. 2019. PMID: 31266861 Free PMC article.
-
Genetic Mapping of Pathogenesis Determinants in Toxoplasma gondii.Annu Rev Microbiol. 2016 Sep 8;70:63-81. doi: 10.1146/annurev-micro-091014-104353. Epub 2016 Jun 17. Annu Rev Microbiol. 2016. PMID: 27359216 Review.
-
Modulation of innate immunity by Toxoplasma gondii virulence effectors.Nat Rev Microbiol. 2012 Nov;10(11):766-78. doi: 10.1038/nrmicro2858. Nat Rev Microbiol. 2012. PMID: 23070557 Free PMC article. Review.
Cited by
-
Lessons from Toxoplasma: Host responses that mediate parasite control and the microbial effectors that subvert them.J Exp Med. 2021 Nov 1;218(11):e20201314. doi: 10.1084/jem.20201314. Epub 2021 Oct 20. J Exp Med. 2021. PMID: 34670268 Free PMC article. Review.
-
Dual RNA-seq reveals no plastic transcriptional response of the coccidian parasite Eimeria falciformis to host immune defenses.BMC Genomics. 2017 Sep 5;18(1):686. doi: 10.1186/s12864-017-4095-6. BMC Genomics. 2017. PMID: 28870168 Free PMC article.
-
Toxoplasma gondii virulence factor ROP1 reduces parasite susceptibility to murine and human innate immune restriction.PLoS Pathog. 2022 Dec 7;18(12):e1011021. doi: 10.1371/journal.ppat.1011021. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36476844 Free PMC article.
-
The Hypervariable Tpr Multigene Family of Theileria Parasites, Defined by a Conserved, Membrane-Associated, C-Terminal Domain, Includes Several Copies with Defined Orthology Between Species.J Mol Evol. 2023 Dec;91(6):897-911. doi: 10.1007/s00239-023-10142-z. Epub 2023 Nov 28. J Mol Evol. 2023. PMID: 38017120 Free PMC article.
-
The hitchhiker's guide to parasite dissemination.Cell Microbiol. 2019 Nov;21(11):e13070. doi: 10.1111/cmi.13070. Epub 2019 Jul 8. Cell Microbiol. 2019. PMID: 31219666 Free PMC article. Review.
References
-
- Dubey JP. 2007. The history and life cycle of Toxoplasma gondii, p 1–18. In Weiss LM, Kim K (), Toxoplasma gondii: the model apicomplexan parasite: perspectives and methods. Elsevier, London, United Kingdom.
-
- Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol 146:286–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

