Emerging Tuberculosis Pathogen Hijacks Social Communication Behavior in the Group-Living Banded Mongoose (Mungos mungo)
- PMID: 27165798
- PMCID: PMC4895101
- DOI: 10.1128/mBio.00281-16
Emerging Tuberculosis Pathogen Hijacks Social Communication Behavior in the Group-Living Banded Mongoose (Mungos mungo)
Erratum in
-
Erratum for Alexander et al., Emerging Tuberculosis Pathogen Hijacks Social Communication Behavior in the Group-Living Banded Mongoose (Mungos mungo).mBio. 2016 Jun 21;7(3):e00921-16. doi: 10.1128/mBio.00921-16. mBio. 2016. PMID: 27329748 Free PMC article. No abstract available.
Abstract
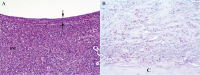
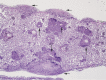
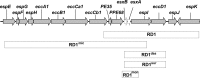
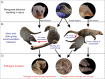
An emerging Mycobacterium tuberculosis complex (MTC) pathogen, M. mungi, infects wild banded mongooses (Mungos mungo) in Northern Botswana, causing significant mortality. This MTC pathogen did not appear to be transmitted through a primary aerosol or oral route. We utilized histopathology, spoligotyping, mycobacterial interspersed repetitive units-variable number of tandem repeats (MIRU-VNTR), quantitative PCR (qPCR), and molecular markers (regions of difference [RDs] from various MTC members, including region of difference 1 [RD1] from M. bovis BCG [RD1(BCG)], M. microti [RD1(mic)], and M. pinnipedii [RD1(seal)], genes Rv1510 [RD4], Rv1970 [RD7], Rv3877/8 [RD1], and Rv3120 [RD12], insertion element IS1561, the 16S RNA gene, and gene Rv0577 [cfp32]), including the newly characterized mongoose-specific deletion in RD1 (RD1(mon)), in order to demonstrate the presence of M. mungi DNA in infected mongooses and investigate pathogen invasion and exposure mechanisms. M. mungi DNA was identified in 29% of nasal planum samples (n = 52), 56% of nasal rinses and swabs (n = 9), 53% of oral swabs (n = 19), 22% of urine samples (n = 23), 33% of anal gland tissue (n = 18), and 39% of anal gland secretions (n = 44). The occurrence of extremely low cycle threshold values obtained with qPCR in anal gland and nasal planum samples indicates that high levels of M. mungi can be found in these tissue types. Histological data were consistent with these results, suggesting that pathogen invasion occurs through breaks in the nasal planum and/or skin of the mongoose host, which are in frequent contact with anal gland secretions and urine during olfactory communication behavior. Lesions in the lung, when present, occurred only with disseminated disease. No environmental sources of M. mungi DNA could be found. We report primary environmental transmission of an MTC pathogen that occurs in association with social communication behavior.
Importance: Organisms causing infectious disease evolve modes of transmission that exploit environmental and host conditions favoring pathogen spread and persistence. We report a novel mode of environmental infectious disease transmission that occurs in association with olfactory secretions (e.g., urine and anal gland secretions), allowing pathogen exposure to occur within and between social groups through intricate social communication behaviors of the banded mongoose host. The presence of M. mungi in these environmentally deposited secretions would effectively circumvent natural social barriers (e.g., territoriality), facilitating between-group pathogen transmission in the absence of direct physical contact, a rare occurrence in this highly territorial species. This work identifies an important potential mechanism of pathogen transmission of epidemiological significance in social species. We also provide evidence of a novel mechanism of pathogen transmission for the MTC complex, where pathogen movement in the environment and host exposure dynamics are driven by social behavior.
Copyright © 2016 Alexander et al.
Figures




Similar articles
-
Pathology of the Emerging Mycobacterium tuberculosis Complex Pathogen, Mycobacterium mungi, in the Banded Mongoose ( Mungos mungo).Vet Pathol. 2018 Mar;55(2):303-309. doi: 10.1177/0300985817741730. Epub 2017 Dec 19. Vet Pathol. 2018. PMID: 29258402
-
Novel Mycobacterium tuberculosis complex pathogen, M. mungi.Emerg Infect Dis. 2010 Aug;16(8):1296-9. doi: 10.3201/eid1608.100314. Emerg Infect Dis. 2010. PMID: 20678329 Free PMC article.
-
The impact of health status on dispersal behavior in banded mongooses (Mungos mungo).Ecohealth. 2014 Jun;11(2):258-62. doi: 10.1007/s10393-014-0912-4. Epub 2014 Feb 7. Ecohealth. 2014. PMID: 24504905
-
Animal-adapted members of the Mycobacterium tuberculosis complex endemic to the southern African subregion.J S Afr Vet Assoc. 2016 Apr 26;87(1):1322. doi: 10.4102/jsava.v87i1.1322. J S Afr Vet Assoc. 2016. PMID: 27246904 Free PMC article. Review.
-
[New era in molecular epidemiology of tuberculosis in Japan].Kekkaku. 2006 Nov;81(11):693-707. Kekkaku. 2006. PMID: 17154049 Review. Japanese.
Cited by
-
Mongoose (Herpestes auropunctatus) May Not Be Reservoir Hosts for Mycobacterium bovis in Fiji Despite High Population Density and Direct Contact with Cattle.Vet Sci. 2019 Oct 24;6(4):85. doi: 10.3390/vetsci6040085. Vet Sci. 2019. PMID: 31652969 Free PMC article.
-
Transmission dynamics and elimination potential of zoonotic tuberculosis in morocco.PLoS Negl Trop Dis. 2017 Feb 2;11(2):e0005214. doi: 10.1371/journal.pntd.0005214. eCollection 2017 Feb. PLoS Negl Trop Dis. 2017. PMID: 28152056 Free PMC article.
-
Pathogenomic analyses of Mycobacterium microti, an ESX-1-deleted member of the Mycobacterium tuberculosis complex causing disease in various hosts.Microb Genom. 2021 Feb;7(2):000505. doi: 10.1099/mgen.0.000505. Microb Genom. 2021. PMID: 33529148 Free PMC article.
-
New insights into the mycobacterial PE and PPE proteins provide a framework for future research.Mol Microbiol. 2020 Jan;113(1):4-21. doi: 10.1111/mmi.14409. Epub 2019 Nov 24. Mol Microbiol. 2020. PMID: 31661176 Free PMC article. Review.
-
Complete genome and transcriptome of Mycobacterium bovis 3488, a clinical isolate with a novel deletion at the RD1 locus.Microbiol Resour Announc. 2025 Apr 10;14(4):e0083724. doi: 10.1128/mra.00837-24. Epub 2025 Mar 10. Microbiol Resour Announc. 2025. PMID: 40062876 Free PMC article.
References
-
- O’Brien DJ, Schmitt SM, Fierke JS, Hogle SA, Winterstein SR, Cooley TM, Moritz WE, Diegel KL, Fitzgerald SD, Berry DE, Kaneene JB. 2002. Epidemiology of Mycobacterium bovis in free-ranging white-tailed deer, Michigan, USA, 1995–2000. Prev Vet Med 54:47–63. doi:10.1016/S0167-5877(02)00010-7. - DOI - PubMed
-
- Courtenay O, Reilly LA, Sweeney FP, Hibberd V, Bryan S, Ul-Hassan A, Newman C, Macdonald DW, Delahay RJ, Wilson GJ, Wellington EM. 2006. Is Mycobacterium bovis in the environment important for the persistence of bovine tuberculosis? Biol Lett 2:460–462. doi:10.1098/rsbl.2006.0468. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

