Induction of Protective Immunity to Cryptococcal Infection in Mice by a Heat-Killed, Chitosan-Deficient Strain of Cryptococcus neoformans
- PMID: 27165801
- PMCID: PMC4959652
- DOI: 10.1128/mBio.00547-16
Induction of Protective Immunity to Cryptococcal Infection in Mice by a Heat-Killed, Chitosan-Deficient Strain of Cryptococcus neoformans
Abstract
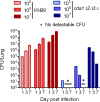
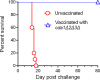
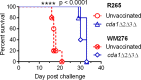
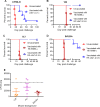
Cryptococcus neoformans is a major opportunistic fungal pathogen that causes fatal meningoencephalitis in immunocompromised individuals and is responsible for a large proportion of AIDS-related deaths. The fungal cell wall is an essential organelle which undergoes constant modification during various stages of growth and is critical for fungal pathogenesis. One critical component of the fungal cell wall is chitin, which in C. neoformans is predominantly deacetylated to chitosan. We previously reported that three chitin deacetylase (CDA) genes have to be deleted to generate a chitosan-deficient C. neoformans strain. This cda1Δ2Δ3Δ strain was avirulent in mice, as it was rapidly cleared from the lungs of infected mice. Here, we report that clearance of the cda1Δ2Δ3Δ strain was associated with sharply spiked concentrations of proinflammatory molecules that are known to be critical mediators of the orchestration of a protective Th1-type adaptive immune response. This was followed by the selective enrichment of the Th1-type T cell population in the cda1Δ2Δ3Δ strain-infected mouse lung. Importantly, this response resulted in the development of robust protective immunity to a subsequent lethal challenge with a virulent wild-type C. neoformans strain. Moreover, protective immunity was also induced in mice vaccinated with heat-killed cda1Δ2Δ3Δ cells and was effective in multiple mouse strains. The results presented here provide a strong framework to develop the cda1Δ2Δ3Δ strain as a potential vaccine candidate for C. neoformans infection.
Importance: The most commonly used anticryptococcal therapies include amphotericin B, 5-fluorocytosine, and fluconazole alone or in combination. Major drawbacks of these treatment options are their limited efficacy, poor availability in limited resource areas, and potential toxicity. The development of antifungal vaccines and immune-based therapeutic interventions is promising and an attractive alternative to chemotherapeutics. Currently, there are no fungal vaccines in clinical use. This is the first report of a C. neoformans deletion strain with an avirulent phenotype in mice exhibiting protective immunity when used as a vaccine after heat inactivation, although other strains that overexpress fungal or murine proteins have recently been shown to induce a protective response. The data presented here demonstrate the potential for developing the avirulent cda1Δ2Δ3Δ strain into a vaccine-based therapy to treat C. neoformans infection.
Copyright © 2016 Upadhya et al.
Figures




References
-
- Byrnes EJ III, Li W, Lewit Y, Ma H, Voelz K, Ren P, Carter DA, Chaturvedi V, Bildfell RJ, May RC, Heitman J. 2010. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog 6:e00547-16. doi:10.1371/journal.ppat.1000850. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
