Endothelin A receptor activation on mesangial cells initiates Alport glomerular disease
- PMID: 27165837
- PMCID: PMC4946972
- DOI: 10.1016/j.kint.2016.02.018
Endothelin A receptor activation on mesangial cells initiates Alport glomerular disease
Abstract
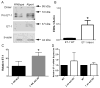
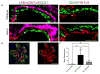
Recent work demonstrates that Alport glomerular disease is mediated through a biomechanical strain-sensitive activation of mesangial actin dynamics. This occurs through a Rac1/CDC42 cross-talk mechanism that results in the invasion of the subcapillary spaces by mesangial filopodia. The filopodia deposit mesangial matrix proteins in the glomerular basement membrane, including laminin 211, which activates focal adhesion kinase in podocytes culminating in the up-regulation of proinflammatory cytokines and metalloproteinases. These events drive the progression of glomerulonephritis. Here we test whether endothelial cell-derived endothelin-1 is up-regulated in Alport glomeruli and further elevated by hypertension. Treatment of cultured mesangial cells with endothelin-1 activates the formation of drebrin-positive actin microspikes. These microspikes do not form when cells are treated with the endothelin A receptor antagonist sitaxentan or under conditions of small, interfering RNA knockdown of endothelin A receptor mRNA. Treatment of Alport mice with sitaxentan results in delayed onset of proteinuria, normalized glomerular basement membrane morphology, inhibition of mesangial filopodial invasion of the glomerular capillaries, normalization of glomerular expression of metalloproteinases and proinflammatory cytokines, increased life span, and prevention of glomerulosclerosis and interstitial fibrosis. Thus endothelin A receptor activation on mesangial cells is a key event in initiation of Alport glomerular disease in this model.
Keywords: Alport syndrome; actin dynamics; endothelin; glomerulonephritis.
Copyright © 2016 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures






Comment in
-
Steps on the Alport path to proteinuria.Kidney Int. 2016 Aug;90(2):242-244. doi: 10.1016/j.kint.2016.03.032. Kidney Int. 2016. PMID: 27418086
References
-
- Kleppel MM, Fan W, Cheong HI, et al. Evidence for separate networks of classical and novel basement membrane collagen. Characterization of alpha 3(IV)-Alport antigen heterodimer. J Biol Chem. 1992;267:4137–4142. - PubMed
-
- Gunwar S, Ballester F, Noelken ME, et al. Glomerular basement membrane. J Biol Chem. 1998;273:8767–8775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

