Endoplasmic reticulum stress and IRE-1 signaling cause apoptosis in colon cancer cells in response to andrographolide treatment
- PMID: 27166181
- PMCID: PMC5173070
- DOI: 10.18632/oncotarget.9180
Endoplasmic reticulum stress and IRE-1 signaling cause apoptosis in colon cancer cells in response to andrographolide treatment
Abstract
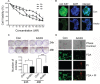
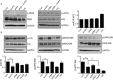
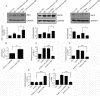
The plant metabolite andrographolide induces cell cycle arrest and apoptosis in cancer cells. The mechanism(s) by which andrographolide induces apoptosis however, have not been elucidated. The present study was performed to determine the molecular events that promote apoptosis in andrographolide treated cells using T84, HCT116 and COLO 205 colon cancer cell lines. Andrographolide was determined to limit colony formation and Ki67 expression, alter nuclear morphology, increase cytoplasmic histone-associated-DNA-fragments, and increase cleaved caspase-3 levels. Andrographolide also induced significantly higher expression of endoplasmic reticulum (ER) stress proteins GRP-78 and IRE-1 by 48 h but not PERK or ATF6. Apoptosis signaling molecules BAX, spliced XBP-1 and CHOP were also significantly increased. Moreover, chemical inhibition of ER stress or IRE-1 depletion with siRNA in andrographolide treated cells significantly limited expression of IRE-1 and CHOP as determined by immunofluorescence staining, real time PCR, or immunobloting. This was accompanied by a decreased BAX/Bcl-2 ratio. Andrographolide significantly promotes cancer cell death compared to normal cells. These data demonstrate that andrographolide associated ER stress contributes to apoptosis through the activation of a pro-apoptotic GRP-78/IRE-1/XBP-1/CHOP signaling pathway.
Keywords: andrographolide; apoptosis; chemotherapy; endoplasmic reticulum stress; unfolded protein response.
Conflict of interest statement
None of the authors participating in this study have any conflicts of interest in this work or publication.
Figures




References
-
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharmaceutics. 2007;4:807–18. - PubMed
-
- Mohanty C, Acharya S, Mohanty AK, Dilnawaz F, Sahoo SK. Curcumin-encapsulated MePEG/PCL diblock copolymeric micelles: a novel controlled delivery vehicle for cancer therapy. Nanomedicine. 2010;5:433–49. - PubMed
-
- Gali-Muhtasib HU, Younes IH, Karchesy JJ, el-Sabban ME. Plant tannins inhibit the induction of aberrant crypt foci and colonic tumors by 1,2-dimethylhydrazine in mice. Nutrition and Cancer. 2001;39:108–16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

