Myosin MyTH4-FERM structures highlight important principles of convergent evolution
- PMID: 27166421
- PMCID: PMC4889382
- DOI: 10.1073/pnas.1600736113
Myosin MyTH4-FERM structures highlight important principles of convergent evolution
Abstract
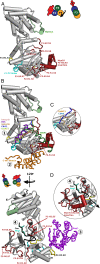
Myosins containing MyTH4-FERM (myosin tail homology 4-band 4.1, ezrin, radixin, moesin, or MF) domains in their tails are found in a wide range of phylogenetically divergent organisms, such as humans and the social amoeba Dictyostelium (Dd). Interestingly, evolutionarily distant MF myosins have similar roles in the extension of actin-filled membrane protrusions such as filopodia and bind to microtubules (MT), suggesting that the core functions of these MF myosins have been highly conserved over evolution. The structures of two DdMyo7 signature MF domains have been determined and comparison with mammalian MF structures reveals that characteristic features of MF domains are conserved. However, across millions of years of evolution conserved class-specific insertions are seen to alter the surfaces and the orientation of subdomains with respect to each other, likely resulting in new sites for binding partners. The MyTH4 domains of Myo10 and DdMyo7 bind to MT with micromolar affinity but, surprisingly, their MT binding sites are on opposite surfaces of the MyTH4 domain. The structural analysis in combination with comparison of diverse MF myosin sequences provides evidence that myosin tail domain features can be maintained without strict conservation of motifs. The results illustrate how tuning of existing features can give rise to new structures while preserving the general properties necessary for myosin tails. Thus, tinkering with the MF domain enables it to serve as a multifunctional platform for cooperative recruitment of various partners, allowing common properties such as autoinhibition of the motor and microtubule binding to arise through convergent evolution.
Keywords: filopodia; microtubules; molecular tinkering; protein evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










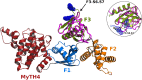
Similar articles
-
Optimized filopodia formation requires myosin tail domain cooperation.Proc Natl Acad Sci U S A. 2019 Oct 29;116(44):22196-22204. doi: 10.1073/pnas.1901527116. Epub 2019 Oct 14. Proc Natl Acad Sci U S A. 2019. PMID: 31611382 Free PMC article.
-
MyTH4-FERM myosins have an ancient and conserved role in filopod formation.Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):E8059-E8068. doi: 10.1073/pnas.1615392113. Epub 2016 Nov 23. Proc Natl Acad Sci U S A. 2016. PMID: 27911821 Free PMC article.
-
The filopodial myosin DdMyo7 is a slow, calcium-regulated motor.J Biol Chem. 2025 May;301(5):108371. doi: 10.1016/j.jbc.2025.108371. Epub 2025 Mar 3. J Biol Chem. 2025. PMID: 40043952 Free PMC article.
-
Myosin-X: a molecular motor at the cell's fingertips.Trends Cell Biol. 2005 Oct;15(10):533-9. doi: 10.1016/j.tcb.2005.08.006. Trends Cell Biol. 2005. PMID: 16140532 Review.
-
Cargo recognition and cargo-mediated regulation of unconventional myosins.Acc Chem Res. 2014 Oct 21;47(10):3061-70. doi: 10.1021/ar500216z. Epub 2014 Sep 17. Acc Chem Res. 2014. PMID: 25230296 Review.
Cited by
-
ROS induced distribution of mitochondria to filopodia by Myo19 depends on a class specific tryptophan in the motor domain.Sci Rep. 2017 Sep 14;7(1):11577. doi: 10.1038/s41598-017-11002-9. Sci Rep. 2017. PMID: 28912530 Free PMC article.
-
Structure of Myo7b/USH1C complex suggests a general PDZ domain binding mode by MyTH4-FERM myosins.Proc Natl Acad Sci U S A. 2017 May 9;114(19):E3776-E3785. doi: 10.1073/pnas.1702251114. Epub 2017 Apr 24. Proc Natl Acad Sci U S A. 2017. PMID: 28439001 Free PMC article.
-
MyTH4-FERM myosins in the assembly and maintenance of actin-based protrusions.Curr Opin Cell Biol. 2017 Feb;44:68-78. doi: 10.1016/j.ceb.2016.10.002. Epub 2016 Nov 9. Curr Opin Cell Biol. 2017. PMID: 27836411 Free PMC article. Review.
-
Myosin 15 participates in assembly and remodeling of the presynapse.J Cell Biol. 2025 Sep 1;224(9):e202305059. doi: 10.1083/jcb.202305059. Epub 2025 Jul 8. J Cell Biol. 2025. PMID: 40627464 Free PMC article.
-
Optimized filopodia formation requires myosin tail domain cooperation.Proc Natl Acad Sci U S A. 2019 Oct 29;116(44):22196-22204. doi: 10.1073/pnas.1901527116. Epub 2019 Oct 14. Proc Natl Acad Sci U S A. 2019. PMID: 31611382 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

