Lipid-Bilayer Dynamics Probed by a Carbon Dot-Phospholipid Conjugate
- PMID: 27166809
- PMCID: PMC4939762
- DOI: 10.1016/j.bpj.2016.04.005
Lipid-Bilayer Dynamics Probed by a Carbon Dot-Phospholipid Conjugate
Abstract
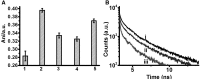
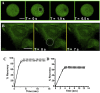
Elucidating the dynamic properties of membranes is important for understanding fundamental cellular processes and for shedding light on the interactions of proteins, drugs, and viruses with the cell surface. Dynamic studies of lipid bilayers have been constrained, however, by the relatively small number of pertinent molecular probes and the limited physicochemical properties of the probes. We show that a lipid conjugate comprised of a fluorescent carbon dot (C-dot) covalently attached to a phospholipid constitutes a versatile and effective vehicle for studying bilayer dynamics. The C-dot-modified phospholipids readily incorporated within biomimetic membranes, including solid-supported bilayers and small and giant vesicles, and inserted into actual cellular membranes. We employed the C-dot-phospholipid probe to elucidate the effects of polymyxin-B (a cytolytic peptide), valproic acid (a lipophilic drug), and amyloid-β (a peptide associated with Alzheimer's disease) upon bilayer fluidity and lipid dynamics through the application of various biophysical techniques.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Pucadyil T.J., Mukherjee S., Chattopadhyay A. Organization and dynamics of NBD-labeled lipids in membranes analyzed by fluorescence recovery after photobleaching. J. Phys. Chem. B. 2007;111:1975–1983. - PubMed
-
- Guo L., Har J.Y., Wohland T. Molecular diffusion measurement in lipid bilayers over wide concentration ranges: a comparative study. ChemPhysChem. 2008;9:721–728. - PubMed
-
- Tero R. Substrate effects on the formation process, structure and physicochemical properties of supported lipid bilayers. Materials (Basel) 2012;5:2658.
-
- Singer S.J., Nicolson G.L. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. - PubMed
-
- Ma Y., Jiang R., Sun X.-L. Chemoselectively surface funtionalizable tethered bilayer lipid membrane for versatile membrane mimetic systems fabrication. J. Mater. Chem. 2012;22:6148–6155.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

