Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models
- PMID: 27167191
- PMCID: PMC5129956
- DOI: 10.18632/oncotarget.9153
Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models
Abstract
Purpose: Though the efficacy of MEK inhibitors is being investigated in KRAS-mutant colorectal cancers (CRC), early clinical trials of MEK inhibitor monotherapy did not reveal significant antitumor activity. Resistance to MEK inhibitor monotherapy developed through a variety of mechanisms converging in ERK reactivation. Since ERK increases cyclin D expression and increases entry into the cell cycle, we hypothesized that the combination of MEK inhibitors and CDK4/6 inhibitors would have synergistic antitumor activity and cause tumor regression in vivo.
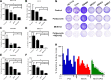
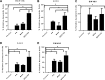
Results: The combination of MEK and CDK4/6 inhibitors synergistically inhibited cancer cell growth in vitro and caused tumor regression in vivo in cell line and patient-derived xenograft models. Combination therapy markedly decreased levels of phosphorylated ribosomal protein S6 both in vitro and in vivo and decreased Ki67 staining in vivo.
Experimental design: We performed in vitro proliferation, colony formation, apoptosis, and senescence assays, and Western blots, on a panel of 11 KRAS mutant CRC cell lines treated with the MEK inhibitor MEK162, the CDK4/6 inhibitor palbociclib, or the combination. We also treated 4 KRAS mutant CRC cell line and patient-derived xenografts with the MEK inhibitor trametinib, the CDK4/6 inhibitor palbociclib, or the combination, and performed immunohistochemical and reverse phase protein array analysis.
Conclusions: Combined inhibition of both MEK and CDK4/6 is effective in preclinical models of KRAS mutant CRC and justifies a planned phase II clinical trial in patients with refractory KRAS-mutant CRC.Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models.
Keywords: CDK4/6 inhibitor; KRAS; MEK inhibitor; NRAS.
Conflict of interest statement
There are no significant conflicts of interest to disclose.
Figures




References
-
- Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

