Mapping General Anesthetic Sites in Heteromeric γ-Aminobutyric Acid Type A Receptors Reveals a Potential For Targeting Receptor Subtypes
- PMID: 27167687
- PMCID: PMC5073028
- DOI: 10.1213/ANE.0000000000001368
Mapping General Anesthetic Sites in Heteromeric γ-Aminobutyric Acid Type A Receptors Reveals a Potential For Targeting Receptor Subtypes
Abstract
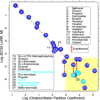
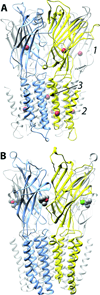
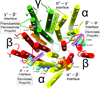
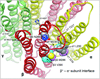
IV general anesthetics, including propofol, etomidate, alphaxalone, and barbiturates, produce important actions by enhancing γ-aminobutyric acid type A (GABAA) receptor activation. In this article, we review scientific studies that have located and mapped IV anesthetic sites using photoaffinity labeling and substituted cysteine modification protection. These anesthetics bind in transmembrane pockets between subunits of typical synaptic GABAA receptors, and drugs that display stereoselectivity also show remarkably selective interactions with distinct interfacial sites. These results suggest strategies for developing new drugs that selectively modulate distinct GABAA receptor subtypes.
Figures


References
-
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. - PubMed
-
- Sieghart W. Allosteric modulation of GABAA receptors via multiple drug-binding sites. Adv Pharmacol. 2015;72:53–96. - PubMed
-
- Miller PS, Smart TG. Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol Sci. 2010;31:161–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

