Stress promotes Arabidopsis - Piriformospora indica interaction
- PMID: 27167761
- PMCID: PMC4973781
- DOI: 10.1080/15592324.2015.1136763
Stress promotes Arabidopsis - Piriformospora indica interaction
Abstract
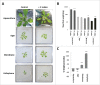
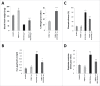
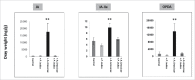
The endophytic fungus Piriformospora indica colonizes Arabidopsis thaliana roots and promotes plant performance, growth and resistance/tolerance against abiotic and biotic stress. Here we demonstrate that the benefits for the plant increase when the two partners are co-cultivated under stress (limited access to nutrient, exposure to heavy metals and salt, light and osmotic stress, pathogen infection). Moreover, physical contact between P. indica and Arabidopsis roots is necessary for optimal growth promotion, and chemical communication cannot replace the physical contact. Lower nutrient availability down-regulates and higher nutrient availability up-regulates the plant defense system including the expression of pathogenesis-related genes in roots. High light, osmotic and salt stresses support the beneficial interaction between the plant and the fungus. P. indica reduces stomata closure and H2O2 production after Alternaria brassicae infection in leaves and suppresses the defense-related accumulation of the phytohormone jasmonic acid. Thus, shifting the growth conditions toward a stress promotes the mutualistic interaction, while optimal supply with nutrients or low stress diminishes the benefits for the plant in the symbiosis.
Keywords: Biophoton; biotic and abiotic stress; defense; light stress; metal resistance; mutualism; osmotic stress; phytohormones; reactive oxygen species; root architecture; salt stress; stomata.
Figures




References
-
- Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 2013; 32(2):429-48 pii:S0734-9750(13)00222-X; PMID:24380797; http://dx.doi.org/10.1016/j.biotechadv.2013.12.005 - DOI - PubMed
-
- Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosytems scales. Ann Rev Plant Biol 2011; 62:227-50; PMID: 21391813; http://dx.doi.org/1081920210.1146/annurev-arplant-042110-103846 - DOI - PubMed
-
- Tinker PBH, Nye PH. Solute movement in the rhizosphere. Oxford, UK: Oxford Univ Press; 2010; p. 464
-
- Khan AG, Kuek C, Chaudhry TM, Khoo C, Hayes WJ. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 2000; 41:197-207; PMID:10819202; http://dx.doi.org/10.1016/S0045-6535(99)00412-9 - DOI - PubMed
-
- Lambers H, Raven JA, Shaver G, Smith SE. Plant nutrient acquisiton strategies change with soil age. Trends Ecol Evol 2008; 23:95-103; PMID:18191280; http://dx.doi.org/10.1016/j.tree.2007.10.008 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
