Fluorogenic Substrates for In Situ Monitoring of Caspase-3 Activity in Live Cells
- PMID: 27168077
- PMCID: PMC4864350
- DOI: 10.1371/journal.pone.0153209
Fluorogenic Substrates for In Situ Monitoring of Caspase-3 Activity in Live Cells
Abstract
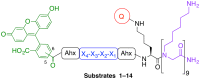
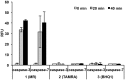
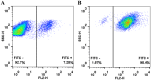
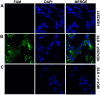
The in situ detection of caspase-3 activity has applications in the imaging and monitoring of multiple pathologies, notably cancer. A series of cell penetrating FRET-based fluorogenic substrates were designed and synthesised for the detection of caspase-3 in live cells. A variety of modifications of the classical caspase-3 and caspase-7 substrate sequence Asp-Glu-Val-Asp were carried out in order to increase caspase-3 affinity and eliminate caspase-7 cross-reactivity. To allow cellular uptake and good solubility, the substrates were conjugated to a cationic peptoid. The most selective fluorogenic substrate 27, FAM-Ahx-Asp-Leu-Pro-Asp-Lys(MR)-Ahx, conjugated to the cell penetrating peptoid at the C-terminus, was able to detect and quantify caspase-3 activity in apoptotic cells without cross-reactivity by caspase-7.
Conflict of interest statement
Figures

References
-
- Tsai FY, Greenbaum DC, Hager JH, Bogyo M, Hanahan D. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 2004; 5: 443–453. - PubMed
-
- Speers AE, Cravatt BF. Profiling enzyme activities in vivo using click chemistry methods. Chem Biol. 2004; 11: 535–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

