Zinc coordination is essential for the function and activity of the type II secretion ATPase EpsE
- PMID: 27168165
- PMCID: PMC5061722
- DOI: 10.1002/mbo3.376
Zinc coordination is essential for the function and activity of the type II secretion ATPase EpsE
Abstract
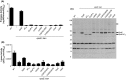
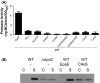
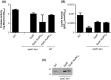
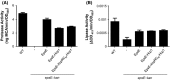
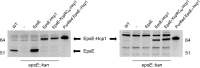
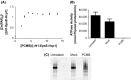
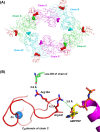
The type II secretion system Eps in Vibrio cholerae promotes the extracellular transport of cholera toxin and several hydrolytic enzymes and is a major virulence system in many Gram-negative pathogens which is structurally related to the type IV pilus system. The cytoplasmic ATPase EpsE provides the energy for exoprotein secretion through ATP hydrolysis. EpsE contains a unique metal-binding domain that coordinates zinc through a tetracysteine motif (CXXCX29 CXXC), which is also present in type IV pilus assembly but not retraction ATPases. Deletion of the entire domain or substitution of any of the cysteine residues that coordinate zinc completely abrogates secretion in an EpsE-deficient strain and has a dominant negative effect on secretion in the presence of wild-type EpsE. Consistent with the in vivo data, chemical depletion of zinc from purified EpsE hexamers results in loss of in vitro ATPase activity. In contrast, exchanging the residues between the two dicysteines with those from the homologous ATPase XcpR from Pseudomonas aeruginosa does not have a significant impact on EpsE. These results indicate that, although the individual residues in the metal-binding domain are generally interchangeable, zinc coordination is essential for the activity and function of EpsE.
Keywords: ATPase; tetracysteine; type II secretion; zinc..
© 2016 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Figures



References
-
- Abendroth, J. , Murphy P., Sandkvist M., Bagdasarian M., and Hol W. G. J.. 2005. The x‐ray structure of the type II secretion system complex formed by the N‐terminal domain of EpsE and the cytoplasmic domain of EpsL of Vibrio cholerae . J. Mol. Biol. 348:845–855. - PubMed
-
- Chiang, P. , Sampaleanu L. M., Ayers M., Pahuta M., Howell P. L., and Burrows L. L.. 2008. Functional role of conserved residues in the characteristic secretion NTPase motifs of the Pseudomonas aeruginosa type IV pilus motor proteins PilB, PilT, and PilU. Microbiology 154:114–126. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

