An AGAMOUS intron-driven cytotoxin leads to flowerless tobacco and produces no detrimental effects on vegetative growth of either tobacco or poplar
- PMID: 27168170
- PMCID: PMC5103258
- DOI: 10.1111/pbi.12581
An AGAMOUS intron-driven cytotoxin leads to flowerless tobacco and produces no detrimental effects on vegetative growth of either tobacco or poplar
Abstract
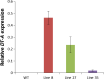
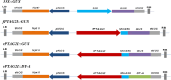
Flowerless trait is highly desirable for poplar because it can prevent pollen- and seed-mediated transgene flow. We have isolated the second intron of PTAG2, an AGAMOUS (AG) orthologue from Populus trichocarpa. By fusing this intron sequence to a minimal 35S promoter sequence, we created two artificial promoters, fPTAG2I (forward orientation of the PTAG2 intron sequence) and rPTAG2I (reverse orientation of the PTAG2 intron sequence). In tobacco, expression of the β-glucuronidase gene (uidA) demonstrates that the fPTAG2I promoter is non-floral-specific, while the rPTAG2I promoter is active in floral buds but with no detectable vegetative activity. Under glasshouse conditions, transgenic tobacco plants expressing the Diphtheria toxin A (DT-A) gene driven by the rPTAG2I promoter produced three floral ablation phenotypes: flowerless, neuter (stamenless and carpel-less) and carpel-less. Further, the vegetative growth of these transgenic lines was similar to that of the wild-type plants. In field trials during 2014 and 2015, the flowerless transgenic tobacco stably maintained its flowerless phenotype, and also produced more shoot and root biomass when compared to wild-type plants. In poplar, the rPTAG2I::GUS gene exhibited no detectable activity in vegetative organs. Under field conditions over two growing seasons (2014 to the end of 2015), vegetative growth of the rPTAG2I::DT-A transgenic poplar plants was similar to that of the wild-type plants. Our results demonstrate that the rPTAG2I artificial promoter has no detectable activities in vegetative tissues and organs, and the rPTAG2I::DT-A gene may be useful for producing flowerless poplar that retains normal vegetative growth.
Keywords: AGAMOUS; Populus; Intron; flowerless; neuter (stamenless and carpel-less); transgene flow.
© 2016 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures



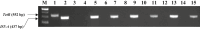


Similar articles
-
Two similar but distinct second intron fragments from tobacco AGAMOUS homologs confer identical floral organ-specific expression sufficient for generating complete sterility in plants.Planta. 2010 Apr;231(5):1159-69. doi: 10.1007/s00425-010-1120-2. Epub 2010 Feb 25. Planta. 2010. PMID: 20182740
-
Transgenic sterility in Populus: expression properties of the poplar PTLF, Agrobacterium NOS and two minimal 35S promoters in vegetative tissues.Tree Physiol. 2006 Apr;26(4):401-10. doi: 10.1093/treephys/26.4.401. Tree Physiol. 2006. PMID: 16414919
-
The second intron of AGAMOUS drives carpel- and stamen-specific expression sufficient to induce complete sterility in Arabidopsis.Plant Cell Rep. 2008 May;27(5):855-63. doi: 10.1007/s00299-008-0511-3. Epub 2008 Feb 7. Plant Cell Rep. 2008. PMID: 18256838
-
The poplar bark storage protein gene (Bspa) promoter is responsive to photoperiod and nitrogen in transgenic poplar and active in floral tissues, immature seeds and germinating seeds of transgenic tobacco.Plant Mol Biol. 2001 Jul;46(4):383-94. doi: 10.1023/a:1010600504740. Plant Mol Biol. 2001. PMID: 11485196
-
Intron-Mediated Enhancement: A Tool for Heterologous Gene Expression in Plants?Front Plant Sci. 2017 Jan 6;7:1977. doi: 10.3389/fpls.2016.01977. eCollection 2016. Front Plant Sci. 2017. PMID: 28111580 Free PMC article. Review.
Cited by
-
Elevated auxin and reduced cytokinin contents in rootstocks improve their performance and grafting success.Plant Biotechnol J. 2017 Dec;15(12):1556-1565. doi: 10.1111/pbi.12738. Epub 2017 May 16. Plant Biotechnol J. 2017. PMID: 28376249 Free PMC article.
-
Identification of candidate genes for leaf scorch in Populus deltoids by the whole genome resequencing analysis.Sci Rep. 2018 Nov 6;8(1):16416. doi: 10.1038/s41598-018-33739-7. Sci Rep. 2018. PMID: 30401919 Free PMC article.
-
Transgenic Reduction of Cytokinin Levels in Roots Inhibits Root-Sprouting in Populus.Plant Physiol. 2019 Aug;180(4):1788-1792. doi: 10.1104/pp.19.00217. Epub 2019 May 31. Plant Physiol. 2019. PMID: 31152128 Free PMC article.
-
Integrated physiological and genomic analysis reveals structural variations and expression patterns of candidate genes for colored- and green-leaf poplar.Sci Rep. 2019 Aug 1;9(1):11150. doi: 10.1038/s41598-019-47681-9. Sci Rep. 2019. PMID: 31371772 Free PMC article.
-
Molecular and physiological characterization of the effects of auxin-enriched rootstock on grafting.Hortic Res. 2021 Apr 1;8(1):74. doi: 10.1038/s41438-021-00509-y. Hortic Res. 2021. PMID: 33790234 Free PMC article.
References
-
- Anand, A. , Zhou, T. , Trick, H.N. , Gill, B.S. , Bockus, W.W. and Muthukrishnan, S. (2003) Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin‐like protein, chitinase and glucanase against Fusarium graminearum. J. Exp. Bot. 54, 1101–1111. - PubMed
-
- Bowman, J.L. , Smyth, D.R. and Meyerowitz, E.M. (1991) Genetic interactions among floral homeotic genes of Arabidopsis. Development, 112, 1–20. - PubMed
-
- Brandle, J. , McHugh, S. , James, L. , Labbe, H. and Miki, B. (1995) Instability of transgene expression in field grown tobacco carrying the csr1‐1 gene for sulfonylurea herbicide resistance. Nat. Biotechnol. 13, 994–998.
-
- Brown, B.J. , Mitchell, R.J. and Graham, S.A. (2002) Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology, 83, 2328–2336.
-
- Brunner, A.M. , Rottmann, W.H. , Sheppard, L.A. , Krutovskii, K. , DiFazio, S.P. , Leonardi, S. and Strauss, S.H. (2000) Structure and expression of duplicate AGAMOUS orthologues in poplar. Plant Mol. Biol. 44, 619–634. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

