Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design
- PMID: 27168247
- PMCID: PMC6034635
- DOI: 10.1146/annurev-immunol-041015-055515
Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design
Abstract
HIV employs multiple means to evade the humoral immune response, particularly the elicitation of and recognition by broadly neutralizing antibodies (bnAbs). Such antibodies can act antivirally against a wide spectrum of viruses by targeting relatively conserved regions on the surface HIV envelope trimer spike. Elicitation of and recognition by bnAbs are hindered by the arrangement of spikes on virions and the relatively difficult access to bnAb epitopes on spikes, including the proximity of variable regions and a high density of glycans. Yet, in a small proportion of HIV-infected individuals, potent bnAb responses do develop, and isolation of the corresponding monoclonal antibodies has been facilitated by identification of favorable donors with potent bnAb sera and by development of improved methods for human antibody generation. Molecular studies of recombinant Env trimers, alone and in interaction with bnAbs, are providing new insights that are fueling the development and testing of promising immunogens aimed at the elicitation of bnAbs.
Keywords: conserved epitopes; immunization strategies; passive immunotherapy; rational vaccine design; vaccination.
Figures
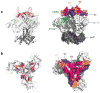



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

