Fermented Milk Consumption and Common Infections in Children Attending Day-Care Centers: A Randomized Trial
- PMID: 27168455
- PMCID: PMC5084641
- DOI: 10.1097/MPG.0000000000001248
Fermented Milk Consumption and Common Infections in Children Attending Day-Care Centers: A Randomized Trial
Abstract
Objectives: This multicenter, double-blind, randomized, placebo-controlled clinical trial investigated the effect of a fermented milk product containing the Lactobacillus casei National Collection of Microorganisms and Cell Cultures (CNCM) I-1518 strain on respiratory and gastrointestinal common infectious diseases (CIDs) in children attending day-care centers in Russia.
Methods: Children ages 3 to 6 years received 100 g of a fermented milk product (n = 300) or a control product (n = 299) twice daily for 3 months, followed by a 1-month observation period. The primary outcome was the incidence of CIDs during the product consumption period.
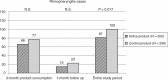
Results: There was no significant difference in the incidence of CIDs between the groups (N = 98 with fermented milk product vs N = 93 with control product). The overall number of CIDs (and no severe cases at all) in both study groups and in all 12 centers, however, was unexpectedly low resulting in underpowering of the study. No differences were found between the groups in the duration or severity of disease, duration of sick leave from day-care centers, parental missed working days, or in quality-of-life dimensions on the PedsQL questionnaire (P > 0.05).There was, however, a significantly lower incidence of the most frequently observed CID, rhinopharyngitis, in children consuming the fermented milk product compared with those consuming the control product (N = 81 vs N = 100, relative risk 0.82, 95% confidence interval 0.69-0.96, P = 0.017) when considering the entire study period.
Conclusions: Although no other significant differences were shown between the fermented milk and control product groups in this study, lower incidence of rhinopharyngitis may indicate a beneficial effect of this fermented milk product.
Trial registration: ClinicalTrials.gov NCT01200173.
Conflict of interest statement
VN was an employee of Danone Research, France, at the time of study conduct. APP received funding from Danone Research, France, as the main investigator of the study. JS received fees for consulting and as speaker at symposia and funds for projects on probiotics, respectively, from Danone, Bauer, Campina, Chr Hansen. Danisco, Dr Fischer, HSO, Merck, Mueller, Nestlé, Orthomol, Wakunaga, and Yakult. The other authors report no conflicts of interest.
Figures

References
-
- McCutcheon H, Fitzgerald M. The public health problem of acute respiratory illness in childcare. J Clin Nurs 2001; 10:305–310. - PubMed
-
- Berdeaux G, Hervié C, Smajda C, et al. Parental quality of life and recurrent ENT infections in their children: development of a questionnaire. Rhinitis Survey Group. Qual Life Res 1998; 6:501–512. - PubMed
-
- Fleming DW, Cochi SL, Hightower AW, et al. Childhood upper respiratory tract infections: to what degree is incidence affected by day-care attendance? Pediatrics 1987; 79:55–60. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

