Interventions for treating hyperemesis gravidarum
- PMID: 27168518
- PMCID: PMC10421833
- DOI: 10.1002/14651858.CD010607.pub2
Interventions for treating hyperemesis gravidarum
Abstract
Background: Hyperemesis gravidarum is a severe form of nausea and vomiting in pregnancy affecting 0.3% to 1.0% of pregnancies, and is one of the most common indications for hospitalization during pregnancy. While a previous Cochrane review examined interventions for nausea and vomiting in pregnancy, there has not yet been a review examining the interventions for the more severe condition of hyperemesis gravidarum.
Objectives: To assess the effectiveness and safety, of all interventions for hyperemesis gravidarum in pregnancy up to 20 weeks' gestation.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register and the Cochrane Complementary Medicine Field's Trials Register (20 December 2015) and reference lists of retrieved studies.
Selection criteria: Randomized controlled trials of any intervention for hyperemesis gravidarum. Quasi-randomized trials and trials using a cross-over design were not eligible for inclusion.We excluded trials on nausea and vomiting of pregnancy that were not specifically studying the more severe condition of hyperemesis gravidarum.
Data collection and analysis: Two review authors independently reviewed the eligibility of trials, extracted data and evaluated the risk of bias. Data were checked for accuracy.
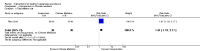
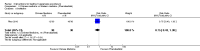
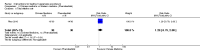
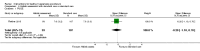
Main results: Twenty-five trials (involving 2052 women) met the inclusion criteria but the majority of 18 different comparisons described in the review include data from single studies with small numbers of participants. The comparisons covered a range of interventions including acupressure/acupuncture, outpatient care, intravenous fluids, and various pharmaceutical interventions. The methodological quality of included studies was mixed. For selected important comparisons and outcomes, we graded the quality of the evidence and created 'Summary of findings' tables. For most outcomes the evidence was graded as low or very low quality mainly due to the imprecision of effect estimates. Comparisons included in the 'Summary of findings' tables are described below, the remaining comparisons are described in detail in the main text.No primary outcome data were available when acupuncture was compared with placebo, There was no clear evidence of differences between groups for anxiodepressive symptoms (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.73 to 1.40; one study, 36 women, very low-quality evidence), spontaneous abortion (RR 0.48, 95% CI 0.05 to 5.03; one study, 57 women, low-quality evidence), preterm birth (RR 0.12, 95% CI 0.01 to 2.26; one study, 36 women, low-quality evidence), or perinatal death (RR 0.57, 95% CI 0.04 to 8.30; one study, 36 women, low-quality evidence).There was insufficient evidence to identify clear differences between acupuncture and metoclopramide in a study with 81 participants regarding reduction/cessation in nausea or vomiting (RR 1.40, 95% CI 0.79 to 2.49 and RR 1.51, 95% CI 0.92 to 2.48, respectively; very low-quality evidence).In a study with 92 participants, women taking vitamin B6 had a slightly longer hospital stay compared with placebo (mean difference (MD) 0.80 days, 95% CI 0.08 to 1.52, moderate-quality evidence). There was insufficient evidence to demonstrate a difference in other outcomes including mean number of episodes of emesis (MD 0.50, 95% CI -0.40 to 1.40, low-quality evidence) or side effects.A comparison between metoclopramide and ondansetron identified no clear difference in the severity of nausea or vomiting (MD 1.70, 95% CI -0.15 to 3.55, and MD -0.10, 95% CI -1.63 to 1.43; one study, 83 women, respectively, very low-quality evidence). However, more women taking metoclopramide complained of drowsiness and dry mouth (RR 2.40, 95% CI 1.23 to 4.69, and RR 2.38, 95% CI 1.10 to 5.11, respectively; moderate-quality evidence). There were no clear differences between groups for other side effects.In a single study with 146 participants comparing metoclopramide with promethazine, more women taking promethazine reported drowsiness, dizziness, and dystonia (RR 0.70, 95% CI 0.56 to 0.87, RR 0.48, 95% CI 0.34 to 0.69, and RR 0.31, 95% CI 0.11 to 0.90, respectively, moderate-quality evidence). There were no clear differences between groups for other important outcomes including quality of life and other side effects.In a single trial with 30 women, those receiving ondansetron had no difference in duration of hospital admission compared to those receiving promethazine (MD 0.00, 95% CI -1.39 to 1.39, very low-quality evidence), although there was increased sedation with promethazine (RR 0.06, 95% CI 0.00 to 0.94, low-quality evidence) .Regarding corticosteroids, in a study with 110 participants there was no difference in days of hospital admission compared to placebo (MD -0.30, 95% CI -0.70 to 0.10; very low-quality evidence), but there was a decreased readmission rate (RR 0.69, 95% CI 0.50 to 0.94; four studies, 269 women). For other important outcomes including pregnancy complications, spontaneous abortion, stillbirth and congenital abnormalities, there was insufficient evidence to identify differences between groups (very low-quality evidence for all outcomes). In other single studies there were no clear differences between groups for preterm birth or side effects (very low-quality evidence).For hydrocortisone compared with metoclopramide, no data were available for primary outcomes and there was no difference in the readmission rate (RR 0.08, 95% CI 0.00 to 1.28;one study, 40 women).In a study with 80 women, compared to promethazine, those receiving prednisolone had increased nausea at 48 hours (RR 2.00, 95% CI 1.08 to 3.72; low-quality evidence), but not at 17 days (RR 0.81, 95% CI 0.58 to 1.15, very low-quality evidence). There was no clear difference in the number of episodes of emesis or subjective improvement in nausea/vomiting. There was insufficient evidence to identify differences between groups for stillbirth and neonatal death and preterm birth.
Authors' conclusions: On the basis of this review, there is little high-quality and consistent evidence supporting any one intervention, which should be taken into account when making management decisions. There was also very limited reporting on the economic impact of hyperemesis gravidarum and the impact that interventions may have.The limitations in interpreting the results of the included studies highlights the importance of consistency in the definition of hyperemesis gravidarum, the use of validated outcome measures, and the need for larger placebo-controlled trials.
Conflict of interest statement
Rupsa C Boelig: none known.
Samantha J Barton: none known.
Gabriele Saccone: none known.
Anthony J Kelly:none known.
Steve J Edwards: none known.
Vincenzo Berghella:none known.
Figures
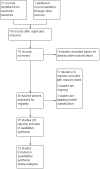

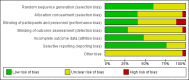





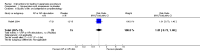






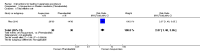










































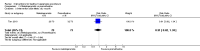
















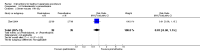












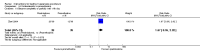
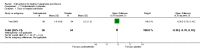
















Update of
References
References to studies included in this review
Abas 2014 {published data only}
-
- Abas MN, Tan PC, Azmi N, Omar SZ. Ondansetron compared with metoclopramide for hyperemesis gravidarum. Obstetrics & Gynecology 2014;123(6):1272‐9. - PubMed
-
- Omar SZ. Ondansetron versus metoclopramide for hyperemesis gravidarum. ISRCTN Registry (http://www.isrctn.com/) [accessed 28 October 2015] 2012.
Bondok 2006 {published data only}
-
- Bondok RS, Sharnouby NM, Eid HE, Abd Elmaksoud AM. Pulsed steroid therapy is an effective treatment for intractable hyperemesis gravidarum. Critical Care Medicine 2006;34(11):2781‐3. - PubMed
Ditto 1999 {published data only}
-
- Ditto A, Morgante G, la‐Marca A, De‐Leo V. Evaluation of treatment of hyperemesis gravidarum using parenteral fluid with or without diazepam. Gynecologic and Obstetric Investigation 1999;48:232‐6. - PubMed
Duggar 2001 {published data only}
-
- Duggar CR, Carlan SJ. The efficacy of methylprednisolone in the treatment of hyperemesis gravidarum: a randomized double‐blind controlled study [abstract]. Obstetrics & Gynecology 2001;97(4 Suppl):45S. - PubMed
Fletcher 2015 {published data only}
-
- Fletcher SJ, Waterman H, Nelson L, Carter LA, Chuang LH, Roberts C, et al. The effectiveness and cost‐effectiveness of a holistic assessment and individualised package of care of women with hyperemesis gravidarum: randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):552‐3.
-
- Fletcher SJ, Waterman H, Nelson L, Carter LA, Dwyer L, Roberts C, et al. Holistic assessment of women with hyperemesis gravidarum: a randomised controlled trial. International Journal of Nursing Studies 2015;52(11):1669‐77. - PubMed
Gawande 2011 {published data only}
-
- Gawande S, Vaidya M, Tadke R, Kirpekar V, Bhave S. Progressive muscle relaxation in hyperemesis gravidarum. Journal of SAFOG 2011;3(1):28‐32.
Habek 2004 {published data only}
-
- Habek D, Barbir A, Habek JC, Janculiak D, Bobic‐Vukovic M. Success of acupuncture and acupressure of the pc 6 acupoint in the treatment of hyperemesis gravidarum. Forschende Komplementarmedizin und Klassische Naturheilkunde 2004;11(1):20‐3. - PubMed
Heazell 2006 {published data only}
-
- Heazell A, Thorneycroft J, Walton V, Etherington I. Acupressure for the in‐patient treatment of nausea and vomiting in early pregnancy: a randomized control trial. American Journal of Obstetrics and Gynecology 2006;194(3):815‐20. - PubMed
Kashifard 2013 {published data only}
-
- Kashifard M, Basirat Z, Kashifard M, Golsorkhtabar‐Amiri M, Moghaddamnia A. Ondansetrone or metoclopromide? Which is more effective in severe nausea and vomiting of pregnancy? A randomized trial double‐blind study. Clinical and Experimental Obstetrics and Gynecology 2013;40(1):127‐30. - PubMed
-
- Pasha NG. A comparison of ondansetron vs. metoclopramide for treatment of nausea and vomiting in pregnancy. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
Mamo 1995 {published data only}
-
- Mamo J, Mamo D, Pace M, Felice D. Evaluation of sea‐band acupressure device for early pregnancy nausea and vomiting. 27th British Congress of Obstetrics And Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:283.
Mao 2010 {published data only}
-
- Mao ZN, Liang CE. Observation on the therapeutic effects of acupuncture on hyperemesis gravidarum. International Journal of Clinical Acupuncture 2010;19(2):60‐5.
-
- Mao ZN, Liang CE. Observation on therapeutic effect of acupuncture on hyperemesis gravidarum. Zhongguo Zhenjiu//Chinese Acupuncture & Moxibustion 2009;29(12):973‐6. - PubMed
McParlin 2008 {published data only}
-
- McParlin C, Carrick‐Sen D, Steen IN, Taylor P, Robson SC. Hyperemesis in pregnancy study: a randomised controlled trial of midwife‐led "outpatient" care. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa9.
-
- Robson SC. Hyperemesis in pregnancy study (ongoing trial). Current Controlled Trials (www.controlled‐trials.com) (accessed 15 February 2007) 2007.
Miller 2001 {published data only}
-
- Veciana M, Stewart L, Miller H, Slotnick R, Rebarber A, Rosen T. Multicenter randomized controlled trial of nerve stimulation therapy for the relief of nausea and vomiting in pregnancy [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S182. - PubMed
-
- Miller H, Veciana M, Stewart L, Rebarber A, Rosen T, Slotnick R. A multicenter randomized controlled trial of nerve stimulation therapy: subanalysis of severe nausea and vomiting symptoms [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S188.
Nelson‐Piercy 2001 {published data only}
-
- Nelson‐Piercy C, Fayers P, Swiet M. Randomised, double‐blind, placebo‐controlled trial of corticosteroids for the treatment of hyperemesis gravidarum. BJOG: an international journal of obstetrics and gynaecology 2001;108:9‐15. - PubMed
Neri 2005 {published data only}
-
- Neri I, Allais G, Schiapparelli P, Blasi I, Benedetto C, Facchinetti F. Acupuncture versus pharmacological approach to reduce hyperemesis gravidarum discomfort. Minerva Ginecologica 2005;57(4):471‐5. - PubMed
Safari 1998 {published data only}
-
- Safari HR, Fassett MJ, Souter IC, Alsulyman OM, Goodwin TM. The efficacy of methylprednisolone in the treatment of hyperemesis gravidarum: a randomized, double‐blind, controlled study. American Journal of Obstetrics and Gynecology 1998;179(4):921‐4. - PubMed
-
- Safari HR, Fassett MJ, Souter IC, Goodwin TM. Randomized double‐blind trial of methylprednisolone versus promethazine in the treatment of hyperemesis gravidarum. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S60. - PubMed
Shin 2007 {published data only}
-
- Shin HS, Song YA, Seo S. Effect of Nei‐Guan point (P6) acupressure on ketonuria levels, nausea and vomiting in women with hyperemesis gravidarum. Journal of Advanced Nursing 2007;59(5):510‐9. - PubMed
Sullivan 1996 {published data only}
-
- Sullivan CA, Johnson CA, Roach H, Martin RW, Stewart DK, Morrison JC. A pilot study of intravenous ondansetron for hyperemesis gravidarum. American Journal of Obstetrics and Gynecology 1996;174(5):1565‐8. - PubMed
-
- Sullivan CA, Johnson CA, Roach H, Martin RW, Stewart DK, Morrison JC. A prospective, randomized, double‐blind comparison of the serotonin antagonist ondansetron to a standardized regimen of promethazine for hyperemesis gravidarum. A preliminary investigation. American Journal of Obstetrics and Gynecology 1995;172:299.
Tabatabaii 2008 {published data only}
-
- Tabatabaii A, Sekhavat L, Mojibian M. A randomized, placebo‐controlled trial of corticosteroids for hyperemesis gravidarum. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):225.
Tan 2009 {published data only}
-
- Tan PC, Yow CM, Omar SZ. A placebo‐controlled trial of oral pyridoxine in hyperemesis gravidarum. Gynecologic and Obstetric Investigation 2009;67(3):151‐7. - PubMed
Tan 2010 {published data only}
-
- Tan PC, Khine PP, Vallikkannu N, Omar SZ. Promethazine compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstetrics & Gynecology 2010;115(5):975‐81. - PubMed
Tan 2013 {published data only}
-
- Tan PC, Norazilah MJ, Omar SZ. Dextrose saline compared with normal saline rehydration of hyperemesis gravidarum: a randomized controlled trial. Obstetrics and Gynecology 2013;121(2 Pt 1):291‐8. - PubMed
Ylikorkala 1979 {published data only}
-
- Ylikorkala O, Kauppila A, Ollanketo ML. Intramuscular ACTH and placebo in the treatment of hyperemesis gravidarum. Acta Obstetricia et Gynecologica Scandinavica 1979;58:453‐5. - PubMed
Yost 2003 {published data only}
-
- Yost NP, McIntire DD, Wians FH, Ramin SM, Balko JA, Leveno KH. A randomized, placebo‐controlled trial of corticosteroids for hyperemesis due to pregnancy. Obstetrics & Gynecology 2003;102(6):1250‐4. - PubMed
Ziaei 2004 {published data only}
-
- Ziaei S, Hosseiney FS, Faghihzadeh S. The efficacy low dose of prednisolone in the treatment of hyperemesis gravidarum. Acta Obstetricia et Gynecologica Scandinavica 2004;83:272‐5. - PubMed
References to studies excluded from this review
Adamczak 2007 {published data only}
-
- Adamczak J, Kasdaglis J, Rinehart B, Antebi Y, Wolf E, Terrone D. A prospective randomized trial of solumedrol dose pack vs. phenergan for the treatment of symptomatic nausea and vomiting in pregnancy. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S88, Abstract no: 277.
Carlsson 2000 {published data only}
-
- Carlsson CP, Axemo P, Bodin A, Carstensen H, Ehrenroth B, Madegard‐Lind I, et al. Manual acupuncture reduces hyperemesis gravidarum: a placebo‐controlled, randomized, single‐blind, crossover study. Journal of Pain & Symptom Management 2000;20(4):273‐9. - PubMed
Dehkordi 2013 {published data only}
-
- Dehkordi EJ. Comparison of "Cydonia Oblonga" fruit product with B6 on nausea and vomiting in pregnancy. IRCT: Iranian Register of Clinical Trials 2013.
Erez 1971 {published data only}
-
- Erez S, Schifrin BS, Dirim O. Double‐blind evaluation of hydroxyzine as an antiemetic in pregnancy. Journal of Reproductive Medicine 1971;7:57‐9. - PubMed
Ferruti 1982 {published data only}
-
- Ferruti MM, Speranza R. Use of the combination of adrenal cortex extract, pyridoxal‐5‐phosphate, cyanocobalamin and ascorbic acid in the treatment of hypocorticism in pregnancy [Italian] [Sulla utilita dell'associazione di estratto corticosurrenale, piridossal‐5‐fosfato, cianocobalamina e acido ascorbico nel trattamento degli stati di ipocorticalismo in gravidanza.]. Minerva Ginecologica 1982;34:619‐23. - PubMed
Fischer‐Rasmussen 1991 {published data only}
-
- Fischer‐Rasmussen W, Kjaer SK, Dahl C, Asping U. Ginger treatment of hyperemesis gravidarum. European Journal of Obstetrics & Gynecology and Reproductive Biology 1991;38(1):19‐24. - PubMed
Ghahiri 2011 {published data only}
-
- Ghahiri AA, Abdi F, Mastoo R, Ghasemi M. The effect of ondansetron and metoclopramide in nausea and vomiting of pregnancy. Journal of Isfahan Medical School 2011; Vol. 29, issue 131.
Gordon 2013 {published data only}
-
- Gordon GH. Dextrose saline compared with normal saline rehydration of hyperemesis gravidarum: a randomized controlled trial. Obstetrics and Gynecology 2013;121(6):1360. - PubMed
Kadan 2009 {published data only}
-
- Kadan Y. Does thiamine help vomiting and nausea in pregnancy?. ClinicalTrials.gov (http://clinicaltrials.gov) (accessed 31 July 2009) 2009.
Koren 2006 {published data only}
-
- Koren G. Pre‐emptive treatment of severe nausea and vomiting of pregnancy (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006) 2006.
Koren 2010 {published data only}
-
- Koren G, Clark S, Hankins GD, Caritis SN, Miodovnik M, Umans JG, et al. Effectiveness of delayed‐release doxylamine and pyridoxine for nausea and vomiting of pregnancy: a randomized placebo controlled trial. American Journal of Obstetrics and Gynecology 2010;203(6):571.e1‐7. - PubMed
Koren 2013 {published data only}
-
- Koren G, Maltepe C. Preemptive Diclectin therapy for the management of nausea and vomiting of pregnancy and hyperemesis gravidarum. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S20.
Koren 2015 {published data only}
Lask 1953 {published data only}
Ling 1994 {published data only}
-
- Ling KM, Dalton M. Progesterone and hyperemesis. British Journal of Family Planning 1994;19(Suppl):9‐10.
Liu 1994 {published data only}
-
- Liu RF, Chen XQ. Treatment of severe pregnant vomiting with mainly self‐made Zengye Ding'ou Tang in 68 cases. Chinese Journal of Integrated Traditional and Western Medicine 1994;14(11):692‐3.
Madegard‐Linh 2004 {published data only}
-
- Madegard‐Linh I. Manual acupuncture reduces hyperemesis gravidarum: a placebo‐controlled, randomized, single‐blind, crossover study. 10th International Conference of Maternity Care Researchers; 2004 June 13‐16; Lund, Sweden. 2004. - PubMed
Magee 1996 {published data only}
-
- Magee LA, Redman CW. An N‐of‐1 trial for treatment of hyperemesis gravidarum. British Journal of Obstetrics and Gynaecology 1996;103(5):478‐80. - PubMed
Maina 2012 {published data only}
-
- Maina A. Transdermal clonidine in the treatment of severe hyperemesis gravidarum (clonemesi). ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 May 2013] 2012.
Maina 2014 {published data only}
-
- Maina A, Arrotta M, Cicogna L, Donvito V, Mischinelli M, Todros T, et al. Transdermal clonidine in the treatment of severe hyperemesis. A pilot randomised control trial: CLONEMESI. BJOG: an International Journal of Obstetrics and Gynaecology 2014;121(12):1556‐63. - PubMed
Maltepe 2012 {published data only}
-
- Maltepe C, Koren G. Pre‐emptive diclectin treatment for nausea and vomiting of pregnancy: Results of a randomized controlled trial. Birth Defects Research Part A ‐ Clinical and Molecular Teratology 2012;94(5):327.
Matok 2013 {published data only}
-
- Matok I, Umans J, Feghali MN, Clark S, Caritis S, Miodovnik M, et al. Characteristics of women with nausea and vomiting of pregnancy who chose to continue compassionate use of placebo after a randomised trial. Journal of Obstetrics & Gynaecology 2013;33(6):557‐60. - PubMed
Matok 2014 {published data only}
-
- Matok I, Clark S, Caritis S, Miodovnik M, Umans G, Hankins G, et al. Studying the antiemetic effect of vitamin B6 for morning sickness: Pyridoxine and pyridoxal are prodrugs. Journal of Clinical Pharmacology 2014;54(12):1429‐33. - PubMed
McCarthy 2014b {published data only}
-
- Higgins JR. Day care versus inpatient management of nausea and vomiting of pregnancy ‐ D.I.M. trial. Current Controlled Trials (http://controlled‐trials.com/) (accessed 5 January 2009) 2009.
-
- Higgins JR. Randomized controlled trial of day care versus inpatient management of nausea and vomiting of pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 May 2013) 2008.
-
- McCarthy FP, Murphy A, Khashan AS, McElroy B, Spillane N, Marchocki Z, et al. Day care compared with inpatient management of nausea and vomiting of pregnancy: a randomized controlled trial. Obstetrics & Gynecology 2014;124(4):743‐8. - PubMed
Nguyen 2008 {published data only}
-
- Nguyen H. The efficacy of diclectin for nausea and vomiting of pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008) 2008.
Oliveira 2014 {published data only}
-
- Capp S, Oliveira L, Carstairs S, You W. Ondansetron versus doxylamine/pyridoxine for treatment of nausea and vomiting in pregnancy: a prospective randomized double‐blind trial. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S39.
-
- Oliveira LG, Capp SM, You WB, Riffenburgh RH, Carstairs SD. Ondansetron compared with Doxylamine and Pyridoxine for treatment of nausea in pregnancy: a randomized controlled trial. Obstetrics and Gynecology 2014;124(4):735‐42. - PubMed
Ozgoli 2009 {published data only}
-
- Ozgoli G, Goli M, Simbar M. Effects of ginger capsules on pregnancy, nausea, and vomiting. Journal of Alternative and Complementary Medicine 2009;15(3):243‐6. - PubMed
Ozgoli 2011 {published data only}
-
- Ozgoli G. Study of Cardamom powder effect on the severity of nausea and vomiting in pregnant women referred to health centers in Chalus city 1389‐90. IRCT Iranian Registry of Clinical Trials (www.irct.ir) [accessed 1 March 2014] 2011.
Price 1964 {published data only}
-
- Price JJ, Barry MC. A double blind study of fluphenazine with pyridoxine. Pennsylvania Medical Journal 1964;67:37‐40. - PubMed
Rad 2010 {published data only}
-
- Rad MN. Evaluation of the influence of KID 21 (youmen) point acupressure on nausea and vomiting of pregnancy. IRCT Iranian Registry of Clinical Trials (www.irct.ir) [accessed 6 December 2010] 2010.
Rosen 2003 {published data only}
-
- Rosen T, Veciana M, Miller HS, Stewart L, Rebarber A, Slotnick RN. A randomized controlled trial of nerve stimulation for relief of nausea and vomiting in pregnancy. Obstetrics & Gynecology 2003;102(1):129‐35. - PubMed
Shin 2005 {published data only}
-
- Shin HS, Song YA. The effect of P6 acupressure for symptom control in pregnant women having hyperemesis gravidarum. Journal of Korean Academy of Nursing 2005;35(3):593‐601. - PubMed
Weiner 1990 {published data only}
-
- Weiner CP. Trial to evaluate the efficacy of promethazine vs metoclopramine in the treatment of hyperemesis gravidarum. Personal communication 1990.
Wibowo 2012 {published data only}
-
- Wibowo N, Purwosunu Y, Sekizawa A, Farina A, Tambunan V, Bardosono S. Vitamin B(6) supplementation in pregnant women with nausea and vomiting. International Journal of Gynecology and Obstetrics 2012;116(3):206‐10. - PubMed
Willetts 2003 {published data only}
-
- Willetts KE, Ekangaki A, Eden JA. Effect of a ginger extract on pregnancy‐induced nausea: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43:139‐44. - PubMed
References to studies awaiting assessment
Eftekhari 2013 {published data only}
-
- Eftekhari N, Mehralhasani Y. A comparison of ondansetron and promethasin in treating hyperemesis gravidarum. Journal of Kerman University of Medical Sciences 2013;20(4):354‐65.
He 2009 {published data only}
-
- He XL, Zhong G, He Y. Clinical observation on treatment of hyperemesis gravidarum by integrative Chinese and Western medicine and its influence on serum motilin. Chinese Journal of Integrated Traditional and Western Medicine 2009;29(10):872‐4. - PubMed
References to ongoing studies
Cyna 2008 {published data only}
-
- Cyna A. The effects of antenatal hypnosis on suffering associated with hyperemesis in early pregnancy. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 19 February 2008) 2008.
Guttuso 2014 {published data only}
-
- Guttuso T. Comparison of gabapentin and metoclopramide for treating hyperemesis gravidarum. ClinicalTrials.gov (http://clinicaltrials.gov) [accessed 28 October 2015] 2014.
Koren 2014 {published data only}
-
- Koren G, Hankins G. A multicenter trial of the efficacy and safety of Diclegis® for nausea and vomiting of pregnancy in pregnant adolescents. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 5 February 2014] 2014.
Mehrolhasani 2012 {published data only}
-
- Mehrolhasani Y. Comparison of Demitron and promethazin in treatment of hyperemesis gravidarum. Iranian Registry of Clinical Trials (accessed 22 July 2013) 2012.
Mitchell‐Jones 2014 {published data only}
-
- Mitchell‐Jones N. Hyperemesis In Pregnancy (HIP) Trial: Inpatient versus outpatient management of severe nausea and vomiting in pregnancy. ISRCTN Registry (http://www.isrctn.com/) [accessed 28 October 2015] 2014.
Additional references
ACOG 2004
-
- American College of Obstetrics and Gynecology. Nausea and Vomiting of Pregnancy. ACOG Practice Bulletin April 2004:1‐12. - PubMed
Arsenault 2002
-
- Arsenault M, Lane CA. The management of nausea and vomiting of pregnancy. Journal of Obsetrics and Gynaecology Canada 2002;24(10):817‐23. - PubMed
Bailit 2005
-
- Bailit JL. Hyperemesis gravidarum: epidemiologic findings from a large cohort. American Journal of Obstetrics and Gynecology 2005;193:811‐4. - PubMed
Bown 2008
-
- Bowen A, Bowen R, Maslany G, Muhajarine N. Anxiety in a socially high‐risk sample of pregnant women in Canada. Canadian Journal of Psychiatry 2008;53:435‐40. - PubMed
Cox 1987
-
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10‐item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry 1987;150:782‐6. - PubMed
Dodds 2006
-
- Dodds L, Fell DB, Joseph KS, Allen VM, Butler B. Outcomes of pregnancies complicated by hyperemesis gravidarum. Obstetrics and Gynecology 2006;107:285‐92. - PubMed
Eliakim 2000
-
- Eliakim R, Abulafia O, Sherer DM. Hyperemesis gravidarum: a current review. American Journal of Perinatology 2000;17(4):207‐18. - PubMed
Fell 2006
-
- Fell DB, Dodds L, Joseph KS, Allen VM, Butler B. Risk factors for hyperemesis gravidarum requiring hospital admission during pregnancy. Obstetrics and Gynecology 2006;107:277‐84. - PubMed
Gadsby 1993
Gill 2009
-
- Gill SK, O'Brien L, Koren G. The safety of histamine 2 blockers in pregnancy: a meta‐analysis. Digestive Diseases and Sciences 2009;54(9):1835‐8. - PubMed
Goodwin 1998
-
- Goodwin TM. Hyperemesis gravidarum. Clinical Obstetrics and Gynecology 1998;41:597‐605. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holmgren 2008
-
- Holmgren C, Aagaard‐Tillery KM, Silver RM, Porter TF, Varner M. Hyperemesis in pregnancy: an evaluation of treatment strategies with maternal and neonatal outcomes. American Journal of Obstetrics and Gynecology 2008;198(1):56.e1‐4. - PubMed
Ismail 2007
-
- Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Practice & Research Clinical Gastroenterology 2007;21(5):755‐69. - PubMed
Jarvis 2011
-
- Jarvis S, Nelson‐Piercy C. Management of nausea and vomiting in pregnancy. BMJ 2011;342:1407‐12. - PubMed
Kelly 2009
-
- Kelly TF, Savides TJ. Gastrointestinal disease in pregnancy. In: Creasy RK, Resnik R, Iams RD editor(s). Creasy and Resnik’s Maternal‐Fetal Medicine: Principles and Practice. 6th Edition. Philadelphia: Saunders, 2009:1041‐57.
Klebanoff 1985
-
- Klebanoff MA, Koslow PA, Keslow R, Rhoads GG. Epidemiology of vomiting in early pregnancy. Obstetrics and Gynecology 1985;66:612‐6. - PubMed
Koren 2005
-
- Koren G, Piwko C, Ahn E, Boskovic R, Maltepe C, Einarson A. Validation studies of the Pregnancy Unique Quantification of Emesis (PUQE) scores. Journal of Obstetrics and Gynecology 2005;25(3):241‐4. - PubMed
Kramer 2013
-
- Kramer J, Bowen A, Stewart N, Muhajarine N. Nausea and vomiting of pregnancy: prevalence, severity and relation to psychosocial health. American Journal of Maternal/Child Nursing 2013;38(1):21‐7. - PubMed
Maltepe 2013
-
- Maltepe C, Koren G. The management of nausea and vomiting of pregnancy and hyperemesis gravidarum‐ a 2013 update. Journal of Population Therapeutics and Clinical Pharmacology 2013;20(2):e184‐e192. - PubMed
Matthews 2015
Mazzotta 2000
-
- Mazzotta P, Magee LA. A risk‐benefit assessment of pharmacological and non‐pharmacological treatments for nausea and vomiting of pregnancy. Drugs 2000;50(4):781‐800. - PubMed
McCarthy 2014a
Mella 2011
-
- Mella MT. Nausea/vomiting of pregnancy and hyperemesis gravidarum. In: Berghella V editor(s). Maternal‐Fetal Evidence Based Guidelines. 2. New York City, New York USA: Informa Healthcare, 2011:72‐80.
Moran 2002
-
- Moran P, Taylor R. Management of hyperemesis gravidarum: the importance of weight loss as a criterion for steroid therapy. Quarterly Journal of Medicine 2002;95:153‐8. - PubMed
Munch 2011
Neutel 2000
-
- Neutel CI. Variation in rates of hospitalization for excessive vomiting in pregnancy by Benedectin/Dicletin use in Canada. In: Koren G, Basahi R editor(s). Nausea and Vomiting of Pregnancy: State of the Art 2000. Toronto: Motherisk, 2000:54‐9.
Niebyl 2010
-
- Niebyl JR. Nausea and vomiting in pregnancy. New England Journal of Medicine 2010;363(16):1544‐50. - PubMed
Pasternak 2013
-
- Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. New England Journal of Medicine 2013;368(9):814‐23. - PubMed
Philip 2003
-
- Philip B. Hyperemesis gravidarum: a literature review. Wisconsin Medical Journal 2003;102(3):46‐51. - PubMed
Piwko 2007
-
- Piwko C, Ungar WJ, Einarson TR, Wolpin J, Koren G. The weekly cost of nausea and vomiting of pregnancy for women calling the Toronto Motherisk Program. Current Medical Research and Opinion 2007;23(4):833‐40. - PubMed
Pongrojpaw 2007
-
- Pongrojpaw D, Somprasit C, Chanthasenanont A. A randomized comparison of ginger and dimenhydrinate in the treatment of nausea and vomiting in pregnancy. Journal of the Medical Association of Thailand 2007;90(9):1703‐9. - PubMed
Poursharif 2007
-
- Poursharif B, Korst LM, MacGibbon KW, Fejzo JS, Romero R, Goodwin TM. Elective pregnancy termination in a large cohort of women with hyperemesis gravidarum. Contraception 2007;76:451‐5. - PubMed
Poursharif 2008
-
- Poursharif B, Korst LM, Fejzo MS, MacGibbon KW, Romero R, Goodwin TM. The psychosocial burden of hyperemesis gravidarum. Journal of Perinatology 2008;28:176‐81. - PubMed
Power 2009
-
- Power Z, Kitchener H, Campbell M, Waterman H. The Hyperemesis Impact of Symptoms Questionaire: development and validation of a clinical tool. International Journal of Nursing Studies 2009;47(1):67‐77. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 1984
-
- Rhodes VA, Watson PM, Johnson MH. Development of reliable and valid measures of nausea and vomiting. Cancer Nursing 1984;7:33‐41. - PubMed
Rhodes 1999
-
- Rhodes VA, McDaniel RW. The Index of Nausea, Vomiting,and Retching: a new format of the Index of Nausea and Vomiting. Oncology Nursing Forum 1999;26(5):889‐94. - PubMed
Saha 2009
Sonkusare 2008
-
- Sonkusare S. Hyperemesis gravidarum: a review. Medical Journal of Malaysia 2008;63(3):272‐7. - PubMed
Swallow 2004
-
- Swallow BL, Lindow SW, Masson EA, Hay DM. Psychological health in early pregnancy: relationship with nausea and vomiting. Journal of Obstetrics and Gynaecology 2004;24(1):28‐32. - PubMed
Tasci 2009
-
- Tasci Y, Demir B, Dilbaz S, Haberal A. Use of diazepam for hyperemesis gravidarum. Journal of Maternal‐Fetal and Neonatal Medicine 2009;22:353‐6. - PubMed
Zhou 2001
-
- Zhou Q, O'Brien B, Soeken K. Rhodes Index of Nausea and Vomiting‐ Form 2 in pregnant women. A confirmatory factor analysis. Nursing Research 2001;50(4):251‐7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

