Multicenter study for efficacy and safety evaluation of a fixeddose combination gel with adapalen 0.1% and benzoyl peroxide 2.5% (Epiduo® for the treatment of acne vulgaris in Brazilian population
- PMID: 27168522
- PMCID: PMC4840853
- DOI: 10.1590/abd1806-4841.20153969
Multicenter study for efficacy and safety evaluation of a fixeddose combination gel with adapalen 0.1% and benzoyl peroxide 2.5% (Epiduo® for the treatment of acne vulgaris in Brazilian population
Abstract
Background: The current options for the treatment of acne vulgaris present many mechanisms of action. For several times, dermatologists try topical agents combinations, looking for better results.
Objectives: To evaluate the efficacy, tolerability and safety of a topical, fixed-dose combination of adapalene 0.1% and benzoyl peroxide 2.5% gel for the treatment of acne vulgaris in the Brazilian population.
Methods: This is a multicenter, open-label and interventionist study. Patients applied 1.0 g of the fixed-dose combination of adapalene 0.1% and benzoyl peroxide 2.5% gel on the face, once daily at bedtime, during 12 weeks. Lesions were counted in all of the appointments, and the degree of acne severity, overall improvement, tolerability and safety were evaluated in each visit.
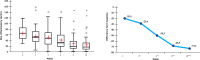
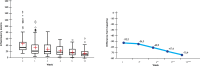
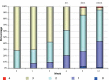
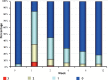
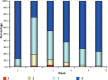
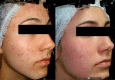
Results: From 79 recruited patients, 73 concluded the study. There was significant, fast and progressive reduction of non-inflammatory, inflammatory and total number of lesions. At the end of the study, 75.3% of patients had a reduction of >50% in non-inflammatory lesions, 69.9% in inflammatory lesions and 78.1% in total number of lesions. Of the 73 patients, 71.2% had good to excellent response and 87.6% had satisfactory to good response. In the first week of treatment, erythema, burning, scaling and dryness of the skin were frequent complaints, but, from second week on, these signals and symptoms have reduced.
Conclusion: The fixed-dose combination of adapalene 0.1% and benzoyl peroxide 2.5% gel is effective, safe, well tolerated and apparently improves patient compliance with the treatment.
Conflict of interest statement
Conflict of interest: Researchers received financial support from the company Galderma.
Figures





References
-
- Prevalence, morbidity, and cost of dermatologic diseases. J Invest Dermatol. 1979;73:395–401. - PubMed
-
- Pawin H, Beylot C, Chivot M, Faure M, Poli F, Revuz J, et al. Physiopathology of acne vulgaris:recent data, new understanding of the treatments. Eur J Dermatol. 2004;14:4–12. - PubMed
-
- Leyden JJ. A review of the use of combination therapies for the treatment of acne vulgaris. J Am Acad Dermatol. 2003;49:S200–S210. - PubMed
-
- Brogden RN, Goa KE. Adapalene: a review of its pharmacological properties and clinical potential in the management of mild to moderate acne. Drugs. 1997;53:511–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
