Phosphatidylserine-targeting antibodies augment the anti-tumorigenic activity of anti-PD-1 therapy by enhancing immune activation and downregulating pro-oncogenic factors induced by T-cell checkpoint inhibition in murine triple-negative breast cancers
- PMID: 27169467
- PMCID: PMC4864933
- DOI: 10.1186/s13058-016-0708-2
Phosphatidylserine-targeting antibodies augment the anti-tumorigenic activity of anti-PD-1 therapy by enhancing immune activation and downregulating pro-oncogenic factors induced by T-cell checkpoint inhibition in murine triple-negative breast cancers
Abstract
Background: The purpose of this study was to investigate the potential of antibody-directed immunotherapy targeting the aminophospholipid phosphatidylserine, which promotes immunosuppression when exposed in the tumor microenvironment, alone and in combination with antibody treatment towards the T-cell checkpoint inhibitor PD-1 in breast carcinomas, including triple-negative breast cancers.
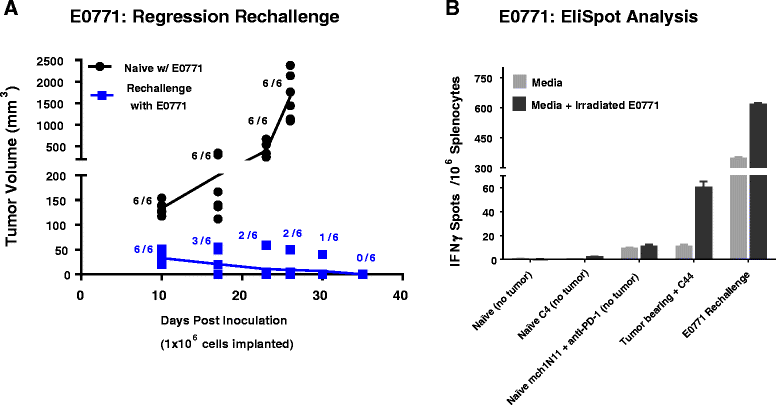
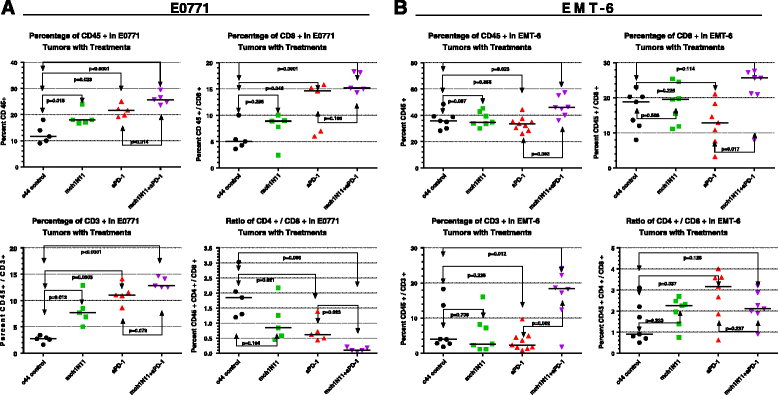
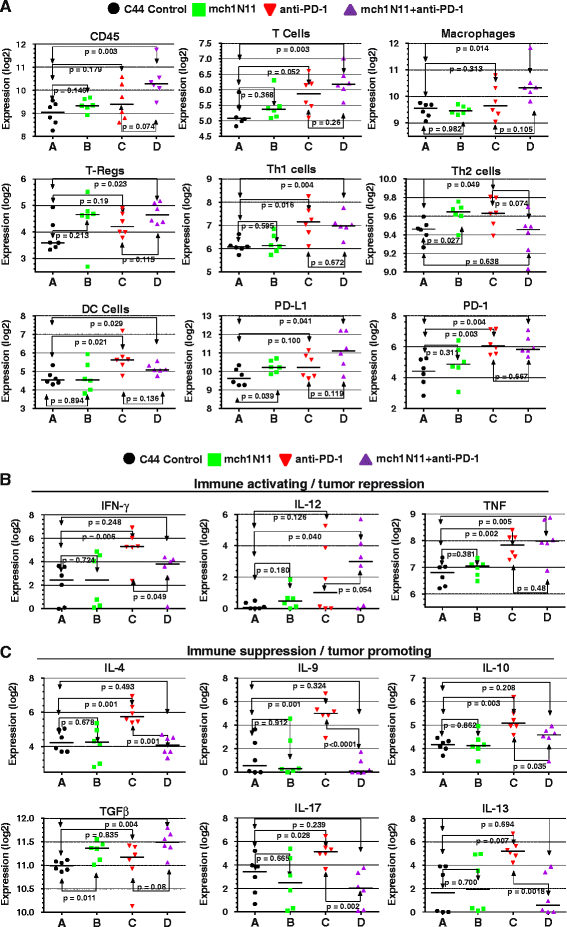
Methods: Immune-competent mice bearing syngeneic EMT-6 or E0771 tumors were subjected to treatments comprising of a phosphatidylserine-targeting and an anti-PD-1 antibody either as single or combinational treatments. Anti-tumor effects were determined by tumor growth inhibition and changes in overall survival accompanying each treatment. The generation of a tumor-specific immune response in animals undergoing complete tumor regression was assessed by secondary tumor cell challenge and splenocyte-produced IFNγ in the presence or absence of irradiated tumor cells. Changes in the presence of tumor-infiltrating lymphocytes were assessed by flow cytometry, while mRNA-based immune profiling was determined using NanoString PanCancer Immune Profiling Panel analysis.
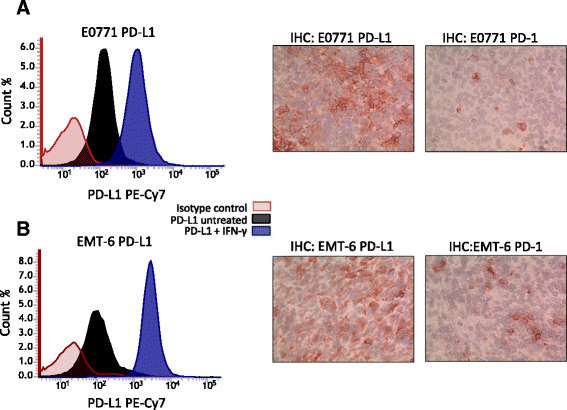
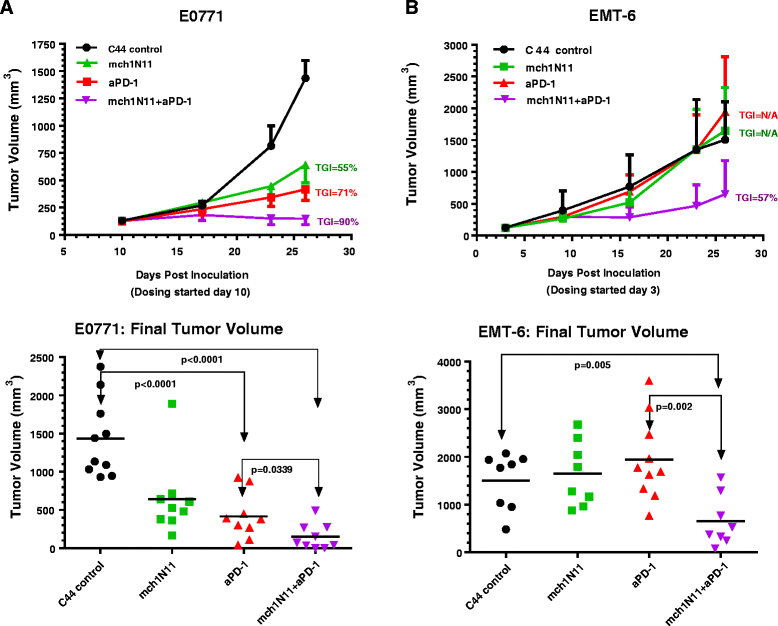
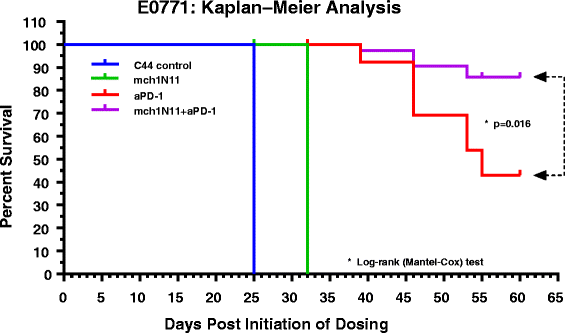
Results: Treatment by a phosphatidylserine-targeting antibody inhibits in-vivo growth and significantly enhances the anti-tumor activity of antibody-mediated PD-1 therapy, including providing a distinct survival advantage over treatment by either single agent. Animals in which complete tumor regression occurred with combination treatments were resistant to secondary tumor challenge and presented heightened expression levels of splenocyte-produced IFNγ. Combinational treatment by a phosphatidylserine-targeting antibody with anti-PD-1 therapy increased the number of tumor-infiltrating lymphocytes more than that observed with single-arm therapies. Finally, immunoprofiling analysis revealed that the combination of anti-phosphatidylserine targeting antibody and anti-PD-1 therapy enhanced tumor-infiltrating lymphocytes, and increased expression of pro-immunosurveillance-associated cytokines while significantly decreasing expression of pro-tumorigenic cytokines that were induced by single anti-PD-1 therapy.
Conclusions: Our data suggest that antibody therapy targeting phosphatidylserine-associated immunosuppression, which has activity as a single agent, can significantly enhance immunotherapies targeting the PD-1 pathway in murine breast neoplasms, including triple-negative breast cancers.
Keywords: Breast cancer; Checkpoint inhibitor; Combination immunotherapy; Phosphatidylserine; Triple-negative breast cancer.
Figures
Similar articles
-
Significance of evaluating tumor-infiltrating lymphocytes (TILs) and programmed cell death-ligand 1 (PD-L1) expression in breast cancer.Med Mol Morphol. 2017 Dec;50(4):185-194. doi: 10.1007/s00795-017-0170-y. Epub 2017 Sep 21. Med Mol Morphol. 2017. PMID: 28936553 Review.
-
Neoadjuvant Interferons: Critical for Effective PD-1-Based Immunotherapy in TNBC.Cancer Immunol Res. 2017 Oct;5(10):871-884. doi: 10.1158/2326-6066.CIR-17-0150. Epub 2017 Aug 28. Cancer Immunol Res. 2017. PMID: 28848054
-
Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts.J Immunother Cancer. 2019 Feb 8;7(1):37. doi: 10.1186/s40425-019-0518-z. J Immunother Cancer. 2019. PMID: 30736857 Free PMC article.
-
Pan-TAM Tyrosine Kinase Inhibitor BMS-777607 Enhances Anti-PD-1 mAb Efficacy in a Murine Model of Triple-Negative Breast Cancer.Cancer Res. 2019 May 15;79(10):2669-2683. doi: 10.1158/0008-5472.CAN-18-2614. Epub 2019 Mar 15. Cancer Res. 2019. PMID: 30877108
-
Immunotherapy for the treatment of breast cancer: checkpoint blockade, cancer vaccines, and future directions in combination immunotherapy.Clin Adv Hematol Oncol. 2016 Nov;14(11):922-933. Clin Adv Hematol Oncol. 2016. PMID: 27930644 Review.
Cited by
-
Efficacy and safety profiles of programmed cell death-1/programmed cell death ligand-1 inhibitors in the treatment of triple-negative breast cancer: A comprehensive systematic review.Oncol Rev. 2019 Oct 10;13(2):425. doi: 10.4081/oncol.2019.425. eCollection 2019 Jul 22. Oncol Rev. 2019. PMID: 31857857 Free PMC article.
-
Bacterial outer membrane vesicle based versatile nanosystem boosts the efferocytosis blockade triggered tumor-specific immunity.Nat Commun. 2023 Mar 25;14(1):1675. doi: 10.1038/s41467-023-37369-0. Nat Commun. 2023. PMID: 36966130 Free PMC article.
-
TAM family kinases as therapeutic targets at the interface of cancer and immunity.Nat Rev Clin Oncol. 2023 Nov;20(11):755-779. doi: 10.1038/s41571-023-00813-7. Epub 2023 Sep 4. Nat Rev Clin Oncol. 2023. PMID: 37667010 Review.
-
Breast cancer lung metastasis: Molecular biology and therapeutic implications.Cancer Biol Ther. 2018;19(10):858-868. doi: 10.1080/15384047.2018.1456599. Epub 2018 Apr 30. Cancer Biol Ther. 2018. PMID: 29580128 Free PMC article. Review.
-
Anticancer therapeutics: a brief account on wide refinements.Am J Cancer Res. 2020 Nov 1;10(11):3599-3621. eCollection 2020. Am J Cancer Res. 2020. PMID: 33294257 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

