Constitutive Intracellular Na+ Excess in Purkinje Cells Promotes Arrhythmogenesis at Lower Levels of Stress Than Ventricular Myocytes From Mice With Catecholaminergic Polymorphic Ventricular Tachycardia
- PMID: 27169737
- PMCID: PMC4902321
- DOI: 10.1161/CIRCULATIONAHA.116.021936
Constitutive Intracellular Na+ Excess in Purkinje Cells Promotes Arrhythmogenesis at Lower Levels of Stress Than Ventricular Myocytes From Mice With Catecholaminergic Polymorphic Ventricular Tachycardia
Abstract
Background: In catecholaminergic polymorphic ventricular tachycardia (CPVT), cardiac Purkinje cells (PCs) appear more susceptible to Ca(2+) dysfunction than ventricular myocytes (VMs). The underlying mechanisms remain unknown. Using a CPVT mouse (RyR2(R4496C+/Cx40eGFP)), we tested whether PC intracellular Ca(2+) ([Ca(2+)]i) dysregulation results from a constitutive [Na(+)]i surplus relative to VMs.
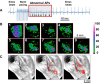
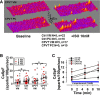
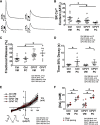
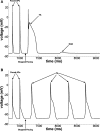
Methods and results: Simultaneous optical mapping of voltage and [Ca(2+)]i in CPVT hearts showed that spontaneous Ca(2+) release preceded pacing-induced triggered activity at subendocardial PCs. On simultaneous current-clamp and Ca(2+) imaging, early and delayed afterdepolarizations trailed spontaneous Ca(2+) release and were more frequent in CPVT PCs than CPVT VMs. As a result of increased activity of mutant ryanodine receptor type 2 channels, sarcoplasmic reticulum Ca(2+) load, measured by caffeine-induced Ca(2+) transients, was lower in CPVT VMs and PCs than respective controls, and sarcoplasmic reticulum fractional release was greater in both CPVT PCs and VMs than respective controls. [Na(+)]i was higher in both control and CPVT PCs than VMs, whereas the density of the Na(+)/Ca(2+) exchanger current was not different between PCs and VMs. Computer simulations using a PC model predicted that the elevated [Na(+)]i of PCs promoted delayed afterdepolarizations, which were always preceded by spontaneous Ca(2+) release events from hyperactive ryanodine receptor type 2 channels. Increasing [Na(+)]i monotonically increased delayed afterdepolarization frequency. Confocal imaging experiments showed that postpacing Ca(2+) spark frequency was highest in intact CPVT PCs, but such differences were reversed on saponin-induced membrane permeabilization, indicating that differences in [Na(+)]i played a central role.
Conclusions: In CPVT mice, the constitutive [Na(+)]i excess of PCs promotes triggered activity and arrhythmogenesis at lower levels of stress than VMs.
Keywords: arrhythmias, cardiac; calcium; calcium signaling; sodium-calcium exchanger.
© 2016 The Authors.
Figures



Similar articles
-
Pharmacological Enhancement of Small Conductance Ca2+-Activated K+ Channels Suppresses Cardiac Arrhythmias in a Mouse Model of Catecholaminergic Polymorphic Ventricular Tachycardia.Circ Res. 2025 Jul 18;137(3):386-399. doi: 10.1161/CIRCRESAHA.124.325477. Epub 2025 May 29. Circ Res. 2025. PMID: 40438932
-
Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation.Cardiovasc Res. 2010 Jul 1;87(1):50-9. doi: 10.1093/cvr/cvq007. Epub 2010 Jan 15. Cardiovasc Res. 2010. PMID: 20080988
-
Purkinje cell calcium dysregulation is the cellular mechanism that underlies catecholaminergic polymorphic ventricular tachycardia.Heart Rhythm. 2010 Aug;7(8):1122-8. doi: 10.1016/j.hrthm.2010.06.010. Epub 2010 Jun 9. Heart Rhythm. 2010. PMID: 20538074 Free PMC article.
-
Ca2+-dependent modulation of voltage-gated myocyte sodium channels.Biochem Soc Trans. 2021 Nov 1;49(5):1941-1961. doi: 10.1042/BST20200604. Biochem Soc Trans. 2021. PMID: 34643236 Free PMC article. Review.
-
Sarcoplasmic Reticulum Ca2+ Dysregulation in the Pathophysiology of Inherited Arrhythmia: An Update.Biochem Pharmacol. 2022 Jun;200:115059. doi: 10.1016/j.bcp.2022.115059. Epub 2022 Apr 29. Biochem Pharmacol. 2022. PMID: 35490731 Review.
Cited by
-
Phosphorylation of the ryanodine receptor 2 at serine 2030 is required for a complete β-adrenergic response.J Gen Physiol. 2019 Feb 4;151(2):131-145. doi: 10.1085/jgp.201812155. Epub 2018 Dec 12. J Gen Physiol. 2019. PMID: 30541771 Free PMC article.
-
Cardiac electrical defects in progeroid mice and Hutchinson-Gilford progeria syndrome patients with nuclear lamina alterations.Proc Natl Acad Sci U S A. 2016 Nov 15;113(46):E7250-E7259. doi: 10.1073/pnas.1603754113. Epub 2016 Oct 31. Proc Natl Acad Sci U S A. 2016. PMID: 27799555 Free PMC article.
-
Delayed afterdepolarization-induced triggered activity in cardiac purkinje cells mediated through cytosolic calcium diffusion waves.Physiol Rep. 2019 Dec;7(24):e14296. doi: 10.14814/phy2.14296. Physiol Rep. 2019. PMID: 31872561 Free PMC article.
-
Purkinje physiology and pathophysiology.J Interv Card Electrophysiol. 2018 Aug;52(3):255-262. doi: 10.1007/s10840-018-0414-3. Epub 2018 Jul 28. J Interv Card Electrophysiol. 2018. PMID: 30056516 Review.
-
Targeted next-generation sequencing provides novel clues for associated epilepsy and cardiac conduction disorder/SUDEP.PLoS One. 2017 Dec 19;12(12):e0189618. doi: 10.1371/journal.pone.0189618. eCollection 2017. PLoS One. 2017. PMID: 29261713 Free PMC article.
References
-
- Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children: a 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. - PubMed
-
- Venetucci L, Denegri M, Napolitano C, Priori SG. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nat Rev Cardiol. 2012;9:561–575. doi: 10.1038/nrcardio.2012.93. - PubMed
-
- Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. - PubMed
-
- Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. - PMC - PubMed
-
- Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, Fauconnier J, Brocard J, Denjoy I, Durand P, Guicheney P, Kyndt F, Leenhardt A, Le Marec H, Lucet V, Mabo P, Probst V, Monnier N, Ray PF, Santoni E, Trémeaux P, Lacampagne A, Fauré J, Lunardi J, Marty I. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum Mol Genet. 2012;21:2759–2767. doi: 10.1093/hmg/dds104. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

