Immunomodulatory Activity of Nivolumab in Metastatic Renal Cell Carcinoma
- PMID: 27169994
- PMCID: PMC5106340
- DOI: 10.1158/1078-0432.CCR-15-2839
Immunomodulatory Activity of Nivolumab in Metastatic Renal Cell Carcinoma
Abstract
Purpose: Nivolumab, an anti-PD-1 immune checkpoint inhibitor, improved overall survival versus everolimus in a phase 3 trial of previously treated patients with metastatic renal cell carcinoma (mRCC). We investigated immunomodulatory activity of nivolumab in a hypothesis-generating prospective mRCC trial.
Experimental design: Nivolumab was administered intravenously every 3 weeks at 0.3, 2, or 10 mg/kg to previously treated patients and 10 mg/kg to treatment-naïve patients with mRCC. Baseline and on-treatment biopsies and blood were obtained. Clinical activity, tumor-associated lymphocytes, PD-L1 expression (Dako immunohistochemistry; ≥5% vs. <5% tumor membrane staining), tumor gene expression (Affymetrix U219), serum chemokines, and safety were assessed.
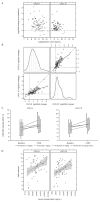
Results: In 91 treated patients, median overall survival [95% confidence interval (CI)] was 16.4 months [10.1 to not reached (NR)] for nivolumab 0.3 mg/kg, NR for 2 mg/kg, 25.2 months (12.0 to NR) for 10 mg/kg, and NR for treatment-naïve patients. Median percent change from baseline in tumor-associated lymphocytes was 69% (CD3+), 180% (CD4+), and 117% (CD8+). Of 56 baseline biopsies, 32% had ≥5% PD-L1 expression, and there was no consistent change from baseline to on-treatment biopsies. Transcriptional changes in tumors on treatment included upregulation of IFNγ-stimulated genes (e.g., CXCL9). Median increases in chemokine levels from baseline to C2D8 were 101% (CXCL9) and 37% (CXCL10) in peripheral blood. No new safety signals were identified.
Conclusions: Immunomodulatory effects of PD-1 inhibition were demonstrated through multiple lines of evidence across nivolumab doses. Biomarker changes from baseline reflect nivolumab pharmacodynamics in the tumor microenvironment. These data may inform potential combinations. Clin Cancer Res; 22(22); 5461-71. ©2016 AACR.
©2016 American Association for Cancer Research.
Figures


References
-
- Buti S, Bersanelli M, Sikokis A, Maines F, Facchinetti F, Bria E, et al. Chemotherapy in metastatic renal cell carcinoma today? A systematic review. Anticancer Drugs. 2013;24:535–54. - PubMed
-
- Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–96. - PubMed
-
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

