Selectivity of Neuromodulatory Projections from the Basal Forebrain and Locus Ceruleus to Primary Sensory Cortices
- PMID: 27170128
- PMCID: PMC6601800
- DOI: 10.1523/JNEUROSCI.4333-15.2016
Selectivity of Neuromodulatory Projections from the Basal Forebrain and Locus Ceruleus to Primary Sensory Cortices
Abstract
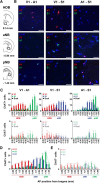
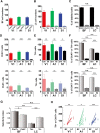
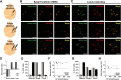
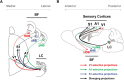
Acetylcholine and noradrenaline are major neuromodulators that affect sensory processing in the cortex. Modality-specific sensory information is processed in defined areas of the cortex, but it is unclear whether cholinergic neurons in the basal forebrain (BF) and noradrenergic neurons in the locus ceruleus (LC) project to and modulate these areas in a sensory modality-selective manner. Here, we mapped BF and LC projections to different sensory cortices of the mouse using dual retrograde tracing. We found that while the innervation of cholinergic neurons into sensory cortices is predominantly modality specific, the projections of noradrenergic neurons diverge onto multiple sensory cortices. Consistent with this anatomy, optogenetic activation of cholinergic neurons in BF subnuclei induces modality-selective desynchronization in specific sensory cortices, whereas activation of noradrenergic LC neurons induces broad desynchronization throughout multiple sensory cortices. Thus, we demonstrate a clear distinction in the organization and function of cholinergic BF and noradrenergic LC projections into primary sensory cortices: cholinergic BF neurons are highly selective in their projections and modulation of specific sensory cortices, whereas noradrenergic LC neurons broadly innervate and modulate multiple sensory cortices.
Significance statement: Neuromodulatory inputs from the basal forebrain (BF) and locus ceruleus (LC) are widespread in the mammalian cerebral cortex and are known to play important roles in attention and arousal, but little is known about the selectivity of their cortical projections. Using a dual retrobead tracing technique along with optogenetic stimulation, we have identified anatomic and functional differences in the way cholinergic BF neurons and noradrenergic LC neurons project into primary sensory cortices. While BF projections are highly selective to individual sensory cortices, LC projections diverge into multiple sensory cortices. To our knowledge, this is the first definitive proof that BF and LC projections to primary sensory cortices show both anatomic and functional differences in selectivity for modulating cortical activity.
Keywords: basal forebrain; cholinergic; locus ceruleus; neuromodulation; noradrenergic; sensory cortex.
Copyright © 2016 the authors 0270-6474/16/365314-14$15.00/0.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
