Restricting nonclassical MHC genes coevolve with TRAV genes used by innate-like T cells in mammals
- PMID: 27170188
- PMCID: PMC4889381
- DOI: 10.1073/pnas.1600674113
Restricting nonclassical MHC genes coevolve with TRAV genes used by innate-like T cells in mammals
Abstract
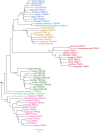
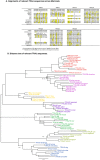
Whereas major histocompatibility class-1 (MH1) proteins present peptides to T cells displaying a large T-cell receptor (TR) repertoire, MH1Like proteins, such as CD1D and MR1, present glycolipids and microbial riboflavin precursor derivatives, respectively, to T cells expressing invariant TR-α (iTRA) chains. The groove of such MH1Like, as well as iTRA chains used by mucosal-associated invariant T (MAIT) and natural killer T (NKT) cells, respectively, may result from a coevolution under particular selection pressures. Herein, we investigated the evolutionary patterns of the iTRA of MAIT and NKT cells and restricting MH1Like proteins: MR1 appeared 170 Mya and is highly conserved across mammals, evolving more slowly than other MH1Like. It has been pseudogenized or independently lost three times in carnivores, the armadillo, and lagomorphs. The corresponding TRAV1 gene also evolved slowly and harbors highly conserved complementarity determining regions 1 and 2. TRAV1 is absent exclusively from species in which MR1 is lacking, suggesting that its loss released the purifying selection on MR1. In the rabbit, which has very few NKT and no MAIT cells, a previously unrecognized iTRA was identified by sequencing leukocyte RNA. This iTRA uses TRAV41, which is highly conserved across several groups of mammals. A rabbit MH1Like gene was found that appeared with mammals and is highly conserved. It was independently lost in a few groups in which MR1 is present, like primates and Muridae, illustrating compensatory emergences of new MH1Like/Invariant T-cell combinations during evolution. Deciphering their role is warranted to search similar effector functions in humans.
Keywords: MAIT; MHC; TCR; evolution; mammals.
Conflict of interest statement
Conflict of interest statement. O.L. received grants from Agence Nationale de la Recherche (ANR) during the study, and received personal fees from NESTEC outside the time of the submitted work.
Figures















References
-
- Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5(6):459–471. - PubMed
-
- Duprat E, Lefranc MP, Gascuel O. A simple method to predict protein-binding from aligned sequences—Application to MHC superfamily and beta2-microglobulin. Bioinformatics. 2006;22(4):453–459. - PubMed
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
-
- Corbett AJ, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509(7500):361–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

