Starch-degrading polysaccharide monooxygenases
- PMID: 27170366
- PMCID: PMC11108391
- DOI: 10.1007/s00018-016-2251-9
Starch-degrading polysaccharide monooxygenases
Abstract
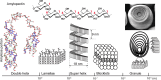
Polysaccharide degradation by hydrolytic enzymes glycoside hydrolases (GHs) is well known. More recently, polysaccharide monooxygenases (PMOs, also known as lytic PMOs or LPMOs) were found to oxidatively degrade various polysaccharides via a copper-dependent hydroxylation. PMOs were previously thought to be either GHs or carbohydrate binding modules (CBMs), and have been re-classified in carbohydrate active enzymes (CAZY) database as auxiliary activity (AA) families. These enzymes include cellulose-active fungal PMOs (AA9, formerly GH61), chitin- and cellulose-active bacterial PMOs (AA10, formerly CBM33), and chitin-active fungal PMOs (AA11). These PMOs significantly boost the activity of GHs under industrially relevant conditions, and thus have great potential in the biomass-based biofuel industry. PMOs that act on starch are the latest PMOs discovered (AA13), which has expanded our perspectives in PMOs studies and starch degradation. Starch-active PMOs have many common structural features and biochemical properties of the PMO superfamily, yet differ from other PMO families in several important aspects. These differences likely correlate, at least in part, to the differences in primary and higher order structures of starch and cellulose, and chitin. In this review we will discuss the discovery, structural features, biochemical and biophysical properties, and possible biological functions of starch-active PMOs, as well as their potential application in the biofuel, food, and other starch-based industries. Important questions regarding various aspects of starch-active PMOs and possible economical driving force for their future studies will also be highlighted.
Keywords: Auxiliary activity family 13; Biofuels; Copper enzymes; Plant pathogens; Polysaccharide monooxygenases; Starch degradation.
Figures



References
-
- Harris PV, Welner D, McFarland KC, Re E, Navarro Poulsen JC, Brown K, Salbo R, Ding H, Vlasenko E, Merino S, Xu F, Cherry J, Larsen S, Lo Leggio L. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: structure and function of a large, enigmatic family. Biochemistry. 2010;49:3305–3316. doi: 10.1021/bi100009p. - DOI - PubMed
-
- Quinlan RJ, Sweeney MD, Lo Leggio L, Otten H, Poulsen JC, Johansen KS, Krogh KB, Jørgensen CI, Tovborg M, Anthonsen A, Tryfona T, Walter CP, Dupree P, Xu F, Davies GJ, Walton PH. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc Natl Acad Sci USA. 2011;108:15079–15084. doi: 10.1073/pnas.1105776108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

