Non-signalling energy use in the developing rat brain
- PMID: 27170699
- PMCID: PMC5322833
- DOI: 10.1177/0271678X16648710
Non-signalling energy use in the developing rat brain
Abstract
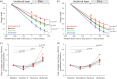
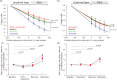
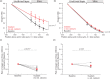
Energy use in the brain constrains its information processing power, but only about half the brain's energy consumption is directly related to information processing. Evidence for which non-signalling processes consume the rest of the brain's energy has been scarce. For the first time, we investigated the energy use of the brain's main non-signalling tasks with a single method. After blocking each non-signalling process, we measured oxygen level changes in juvenile rat brain slices with an oxygen-sensing microelectrode and calculated changes in oxygen consumption throughout the slice using a modified diffusion equation. We found that the turnover of the actin and microtubule cytoskeleton, followed by lipid synthesis, are significant energy drains, contributing 25%, 22% and 18%, respectively, to the rate of oxygen consumption. In contrast, protein synthesis is energetically inexpensive. We assess how these estimates of energy expenditure relate to brain energy use in vivo, and how they might differ in the mature brain.
Keywords: ATP; brain development; brain slice; energy metabolism; lipids.
Figures



References
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001; 21: 1133–1145. - PubMed
-
- Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci 2005; 6: 841–849. - PubMed
-
- Kety S. The general metabolism of the brain in vivo. In: Richter D (ed.) Metabolism of the nervous system, London: Pergamon, 1957, pp. 221–237. .
-
- Sokoloff L. The metabolism of the central nervous system in vivo. In: Field J, Magoun HW and Hall VE (eds) Handbook of physiology-neurophysiology, Washington: American Physiological Society, 1960, pp. 1843–1864. .
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

