Bile Acids Act as Soluble Host Restriction Factors Limiting Cytomegalovirus Replication in Hepatocytes
- PMID: 27170759
- PMCID: PMC4944301
- DOI: 10.1128/JVI.00299-16
Bile Acids Act as Soluble Host Restriction Factors Limiting Cytomegalovirus Replication in Hepatocytes
Abstract
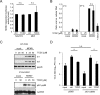
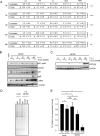
The liver constitutes a prime site of cytomegalovirus (CMV) replication and latency. Hepatocytes produce, secrete, and recycle a chemically diverse set of bile acids, with the result that interactions between bile acids and cytomegalovirus inevitably occur. Here we determined the impact of naturally occurring bile acids on mouse CMV (MCMV) replication. In primary mouse hepatocytes, physiological concentrations of taurochenodeoxycholic acid (TCDC), glycochenodeoxycholic acid, and to a lesser extent taurocholic acid significantly reduced MCMV-induced gene expression and diminished the generation of virus progeny, while several other bile acids did not exert antiviral effects. The anticytomegalovirus activity required active import of bile acids via the sodium-taurocholate-cotransporting polypeptide (NTCP) and was consistently observed in hepatocytes but not in fibroblasts. Under conditions in which alpha interferon (IFN-α) lacks antiviral activity, physiological TCDC concentrations were similarly effective as IFN-γ. A detailed investigation of distinct steps of the viral life cycle revealed that TCDC deregulates viral transcription and diminishes global translation in infected cells.
Importance: Cytomegaloviruses are members of the Betaherpesvirinae subfamily. Primary infection leads to latency, from which cytomegaloviruses can reactivate under immunocompromised conditions and cause severe disease manifestations, including hepatitis. The present study describes an unanticipated antiviral activity of conjugated bile acids on MCMV replication in hepatocytes. Bile acids negatively influence viral transcription and exhibit a global effect on translation. Our data identify bile acids as site-specific soluble host restriction factors against MCMV, which may allow rational design of anticytomegalovirus drugs using bile acids as lead compounds.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Ten Napel CH, Houthoff HJ, The TH. 1984. Cytomegalovirus hepatitis in normal and immune compromised hosts. Liver 4:184–194. - PubMed
-
- Mocarski ES, Shenk T, Pass RF. 2007. Cytomegaloviruses, p 2656–2701. In Knipe DM, Howley PM (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

