Interferon Regulator Factor 8 (IRF8) Limits Ocular Pathology during HSV-1 Infection by Restraining the Activation and Expansion of CD8+ T Cells
- PMID: 27171004
- PMCID: PMC4865128
- DOI: 10.1371/journal.pone.0155420
Interferon Regulator Factor 8 (IRF8) Limits Ocular Pathology during HSV-1 Infection by Restraining the Activation and Expansion of CD8+ T Cells
Abstract
Interferon Regulatory Factor-8 (IRF8) is constitutively expressed in monocytes and B cell lineages and plays important roles in immunity to pathogens and cancer. Although IRF8 expression is induced in activated T cells, the functional relevance of IRF8 in T cell-mediated immunity is not well understood. In this study, we used mice with targeted deletion of Irf8 in T-cells (IRF8KO) to investigate the role of IRF8 in T cell-mediated responses during herpes simplex virus 1 (HSV-1) infection of the eye. In contrast to wild type mice, HSV-1-infected IRF8KO mice mounted a more robust anti-HSV-1 immune response, which included marked expansion of HSV-1-specific CD8+ T cells, increased infiltration of inflammatory cells into the cornea and trigeminal ganglia (TG) and enhanced elimination of virus within the trigeminal ganglion. However, the consequence of the enhanced immunological response was the development of ocular inflammation, limbitis, and neutrophilic infiltration into the cornea of HSV-1-infected IRF8KO mice. Surprisingly, we observed a marked increase in virus-specific memory precursor effector cells (MPEC) in IRF8KO mice, suggesting that IRF8 might play a role in regulating the differentiation of effector CD8+ T cells to the memory phenotype. Together, our data suggest that IRF8 might play a role in restraining excess lymphocyte proliferation. Thus, modulating IRF8 levels in T cells can be exploited therapeutically to prevent immune-mediated ocular pathology during autoimmune and infectious diseases of the eye.
Conflict of interest statement
Figures
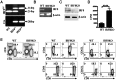

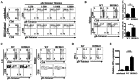
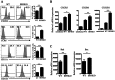


References
-
- Horiuchi M, Wakayama K, Itoh A, Kawai K, Pleasure D, Ozato K, et al. Interferon regulatory factor 8/interferon consensus sequence binding protein is a critical transcription factor for the physiological phenotype of microglia. Journal of neuroinflammation. 2012;9:227 10.1186/1742-2094-9-227 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

