Ultrasound- and Molecular Sieves-Assisted Synthesis, Molecular Docking and Antifungal Evaluation of 5-(4-(Benzyloxy)-substituted phenyl)-3-((phenylamino)methyl)-1,3,4-oxadiazole-2(3H)-thiones
- PMID: 27171073
- PMCID: PMC6273549
- DOI: 10.3390/molecules21050484
Ultrasound- and Molecular Sieves-Assisted Synthesis, Molecular Docking and Antifungal Evaluation of 5-(4-(Benzyloxy)-substituted phenyl)-3-((phenylamino)methyl)-1,3,4-oxadiazole-2(3H)-thiones
Abstract
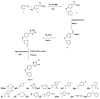
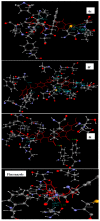
A novel series of 5-(4-(benzyloxy)substituted phenyl)-3-((phenyl amino)methyl)-1,3,4-oxadiazole-2(3H)-thione Mannich bases 6a-o were synthesized in good yield from the key compound 5-(4-(benzyloxy)phenyl)-1,3,4-oxadiazole-2(3H)-thione by aminomethylation with paraformaldehyde and substituted amines using molecular sieves and sonication as green chemistry tools. The antifungal activity of the new products was evaluated against seven human pathogenic fungal strains, namely, Candida albicans ATCC 24433, Candida albicans ATCC 10231, Candida glabrata NCYC 388, Cryptococcus neoformans ATCC 34664, Cryptococcus neoformans PRL 518, Aspergillus fumigatus NCIM 902 and Aspergillus niger ATCC 10578. The synthesized compounds 6d, 6f, 6g, 6h and 6j exhibited promising antifungal activity against the tested fungal pathogens. In molecular docking studies, derivatives 6c, 6f and 6i showed good binding at the active site of C. albicans cytochrome P450 enzyme lanosterol 14 α-demethylase. The in vitro antifungal activity results and docking studies indicated that the synthesized compounds have potential antifungal activity and can be further optimized as privileged scaffolds to design and develop potent antifungal drugs.
Keywords: 1,3,4-oxadiazoles; Mannich reaction; antifungal activity; molecular docking; molecular sieves; ultrasound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Design, synthesis and molecular docking studies of novel triazole as antifungal agent.Eur J Med Chem. 2011 Jul;46(7):3167-76. doi: 10.1016/j.ejmech.2011.04.022. Epub 2011 Apr 15. Eur J Med Chem. 2011. PMID: 21531485
-
Design, synthesis and evaluation of benzoheterocycle analogues as potent antifungal agents targeting CYP51.Bioorg Med Chem. 2018 Jul 23;26(12):3242-3253. doi: 10.1016/j.bmc.2018.04.054. Epub 2018 Apr 27. Bioorg Med Chem. 2018. PMID: 29748145
-
Design, synthesis, and structure-activity relationship studies of benzothiazole derivatives as antifungal agents.Eur J Med Chem. 2016 Nov 10;123:514-522. doi: 10.1016/j.ejmech.2016.07.067. Epub 2016 Jul 29. Eur J Med Chem. 2016. PMID: 27494168
-
The limitless endophytes: their role as antifungal agents against top priority pathogens.Microb Cell Fact. 2024 May 31;23(1):161. doi: 10.1186/s12934-024-02411-3. Microb Cell Fact. 2024. PMID: 38822407 Free PMC article. Review.
-
Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance.Eukaryot Cell. 2008 May;7(5):747-64. doi: 10.1128/EC.00041-08. Epub 2008 Mar 28. Eukaryot Cell. 2008. PMID: 18375617 Free PMC article. Review. No abstract available.
Cited by
-
Green Synthetic Approach: An Efficient Eco-Friendly Tool for Synthesis of Biologically Active Oxadiazole Derivatives.Molecules. 2021 Feb 22;26(4):1163. doi: 10.3390/molecules26041163. Molecules. 2021. PMID: 33671751 Free PMC article. Review.
-
Design, Synthesis, and Biological Activity of New Triazole and Nitro-Triazole Derivatives as Antifungal Agents.Molecules. 2017 Jul 10;22(7):1150. doi: 10.3390/molecules22071150. Molecules. 2017. PMID: 28698522 Free PMC article.
-
Ultrasound Assisted Synthesis of 4-(Benzyloxy)-N-(3-chloro-2-(substitutedphenyl)-4-oxoazetidin-1-yl) Benzamide as Challenging Anti-Tubercular Scaffold.Molecules. 2018 Aug 3;23(8):1945. doi: 10.3390/molecules23081945. Molecules. 2018. PMID: 30081525 Free PMC article.
-
New oxadiazole and pyrazoline derivatives as anti-proliferative agents targeting EGFR-TK: design, synthesis, biological evaluation and molecular docking study.Sci Rep. 2024 Mar 5;14(1):5474. doi: 10.1038/s41598-024-55046-0. Sci Rep. 2024. PMID: 38443456 Free PMC article.
-
Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives.Int J Mol Sci. 2021 Jun 29;22(13):6979. doi: 10.3390/ijms22136979. Int J Mol Sci. 2021. PMID: 34209520 Free PMC article. Review.
References
-
- Nikalje A.G., Ghodke M.S., Khan F.A.K., Sangshetti J.N. CAN catalyzed one-pot synthesis and docking study of some novel substituted imidazole coupled 1,2,4-triazole-5-carboxylic acids as antifungal agents. Chin. Chem. Lett. 2015;26:108–112. doi: 10.1016/j.cclet.2014.10.020. - DOI
-
- Liu F., Luo X.Q., Song B.A., Bhadury P.S., Yang S., Jin L.H., Xue W., Hu D.Y. Synthesis and antifungal activity of novel sulfoxide derivatives containing trimethoxyphenyl substituted 1,3,4-thiadiazole and 1,3,4-oxadiazole moiety. Bioorg. Med. Chem. Lett. 2008;16:3632–3640. doi: 10.1016/j.bmc.2008.02.006. - DOI - PubMed
-
- Chen C.J., Song B.A., Yang S., Xu G.F., Bhadury P.S., Jin L.H., Hu D.Y., Li Q.Z., Liu F., Xue W., et al. Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5-(3,4,5-trimeth-oxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. 2007;15:3981–3989. doi: 10.1016/j.bmc.2007.04.014. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources