The Effect of a High-Fat Diet on Brain Plasticity, Inflammation and Cognition in Female ApoE4-Knockin and ApoE-Knockout Mice
- PMID: 27171180
- PMCID: PMC4865084
- DOI: 10.1371/journal.pone.0155307
The Effect of a High-Fat Diet on Brain Plasticity, Inflammation and Cognition in Female ApoE4-Knockin and ApoE-Knockout Mice
Abstract
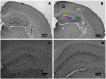
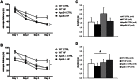
Apolipoprotein E4 (ApoE4), one of three common isoforms of ApoE, is a major risk factor for late-onset Alzheimer disease (AD). ApoE-deficient mice, as well as mice expressing human ApoE4, display impaired learning and memory functions and signs of neurodegeneration. Moreover, ApoE protects against high-fat (HF) diet induced neurodegeneration by its role in the maintenance of the integrity of the blood-brain barrier. The influence of a HF diet on the progression of AD-like cognitive and neuropathological changes was assessed in wild-type (WT), human ApoE4 and ApoE-knockout (ApoE-/-) mice to evaluate the modulatory role of ApoE in this process. From 12 months of age, female WT, ApoE4, and ApoE-/- mice were fed either a standard or a HF diet (19% butter, 0.5% cholate, 1.25% cholesterol) throughout life. At 15 months of age mice performed the Morris water maze, evaluating spatial learning and memory. ApoE-/- showed increased spatial learning compared to WT mice (p = 0.009). HF diet improved spatial learning in WT mice (p = 0.045), but did not affect ApoE4 and ApoE-/- mice. Immunohistochemical analyses of the hippocampus demonstrated increased neuroinflammation (CD68) in the cornu ammonis 1 (CA1) region in ApoE4 (p = 0.001) and in ApoE-/- (p = 0.032) mice on standard diet. HF diet tended to increase CD68 in the CA1 in WT mice (p = 0.052), while it decreased in ApoE4 (p = 0.009), but ApoE-/- remained unaffected. A trend towards increased neurogenesis (DCX) was found in both ApoE4 (p = 0.052) and ApoE-/- mice (p = 0.068). In conclusion, these data suggest that HF intake induces different effects in WT mice compared to ApoE4 and ApoE-/- with respect to markers for cognition and neurodegeneration. We propose that HF intake inhibits the compensatory mechanisms of neuroinflammation and neurogenesis in aged female ApoE4 and ApoE-/- mice.
Conflict of interest statement
Figures

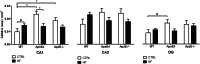


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

