Acneiform Rash Induced by EGFR Inhibitors: Review of the Literature and New Insights
- PMID: 27171241
- PMCID: PMC4857844
- DOI: 10.1159/000371821
Acneiform Rash Induced by EGFR Inhibitors: Review of the Literature and New Insights
Abstract
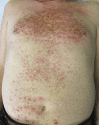
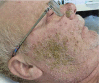
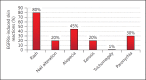
Acneiform rash is the most common side effect of epidermal growth factor receptor (EGFR) inhibitors (EGFRis), and it occurs in 50-100% of patients. This condition can affect the quality of life of these patients and can sometimes lead to a discontinuation of the antineoplastic therapy. Several recent prospective studies have addressed and evaluated different interventions to mitigate or reduce the severity of EGFRis-associated skin rash. With this aim, we have established a dermocosmetological outpatient clinic for cancer patients at the Department of Clinical Medicine and Surgery, University of Naples Federico II in collaboration with the Department of Dermatology and Cutaneous Surgery, Miller School of Medicine, University of Miami. An interdisciplinary network of physicians can improve the quality of life of the cancer patients, focusing on such important aspects as dermocosmetological skin care, but also on the evaluation of new therapeutic and diagnostic algorithms in order to make further progress in the field of prevention. In this review, we summarize the state of the art of the epidemiology, pathogenesis, and treatment of EGFRis acneiform rash, and we describe our outpatient clinical experience.
Keywords: Acneiform rash; EGFR inhibitors; Papulopustular rash.
Figures


References
-
- Wagner L, Lai SE, Aneja M, et al. Development of a functional assessment of side-effects to therapy (FAST) questionnaire to assess dermatology-related quality of life in patients treated with EGFR inhibitors (EGFRI): the FAST-EGFRI (abstract) J Clin Oncol. 2007;25:19532.
-
- Hecht JR, Patnaik A, Berlin J, et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer. 2007;110:980–988. - PubMed
-
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–2246. - PubMed
-
- Lynch TJ, Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610–621. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

