Erastin Disrupts Mitochondrial Permeability Transition Pore (mPTP) and Induces Apoptotic Death of Colorectal Cancer Cells
- PMID: 27171435
- PMCID: PMC4865238
- DOI: 10.1371/journal.pone.0154605
Erastin Disrupts Mitochondrial Permeability Transition Pore (mPTP) and Induces Apoptotic Death of Colorectal Cancer Cells
Abstract
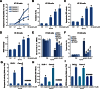
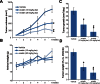
We here evaluated the potential anti-colorectal cancer activity by erastin, a voltage-dependent anion channel (VDAC)-binding compound. Our in vitro studies showed that erastin exerted potent cytotoxic effects against multiple human colorectal cancer cell lines, possibly via inducing oxidative stress and caspase-9 dependent cell apoptosis. Further, mitochondrial permeability transition pore (mPTP) opening was observed in erastin-treated cancer cells, which was evidenced by VDAC-1 and cyclophilin-D (Cyp-D) association, mitochondrial depolarization, and cytochrome C release. Caspase inhibitors, the ROS scavenger MnTBAP, and mPTP blockers (sanglifehrin A, cyclosporin A and bongkrekic acid), as well as shRNA-mediated knockdown of VDAC-1, all significantly attenuated erastin-induced cytotoxicity and apoptosis in colorectal cancer cells. On the other hand, over-expression of VDAC-1 augmented erastin-induced ROS production, mPTP opening, and colorectal cancer cell apoptosis. In vivo studies showed that intraperitoneal injection of erastin at well-tolerated doses dramatically inhibited HT-29 xenograft growth in severe combined immunodeficient (SCID) mice. Together, these results demonstrate that erastin is cytotoxic and pro-apoptotic to colorectal cancer cells. Erastin may be further investigated as a novel anti-colorectal cancer agent.
Conflict of interest statement
Figures



References
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. (2016) Cancer statistics in China, 2015. CA Cancer J Clin. - PubMed
-
- Chen A, Xu J, Johnson AC (2006) Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 25: 278–287. - PubMed
-
- Mohandas KM, Desai DC (1999) Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J Gastroenterol 18: 118–121. - PubMed
-
- Gustin DM, Brenner DE (2002) Chemoprevention of colon cancer: current status and future prospects. Cancer Metastasis Rev 21: 323–348. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

