Quorum Sensing Communication Modules for Microbial Consortia
- PMID: 27172092
- PMCID: PMC5603278
- DOI: 10.1021/acssynbio.5b00286
Quorum Sensing Communication Modules for Microbial Consortia
Abstract
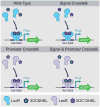
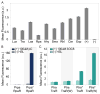
The power of a single engineered organism is limited by its capacity for genetic modification. To circumvent the constraints of any singular microbe, a new frontier in synthetic biology is emerging: synthetic ecology, or the engineering of microbial consortia. Here we develop communication systems for such consortia in an effort to allow for complex social behavior across different members of a community. We posit that such communities will outpace monocultures in their ability to perform complicated tasks if communication among and between members of the community is well regulated. Quorum sensing was identified as the most promising candidate for precise control of engineered microbial ecosystems, due to its large diversity and established utility in synthetic biology. Through promoter and protein modification, we engineered two quorum sensing systems (rpa and tra) to add to the extensively used lux and las systems. By testing the cross-talk between all systems, we thoroughly characterized many new inducible systems for versatile control of engineered communities. Furthermore, we've identified several system pairs that exhibit useful types of orthogonality. Most notably, the tra and rpa systems were shown to have neither signal crosstalk nor promoter crosstalk for each other, making them completely orthogonal in operation. Overall, by characterizing the interactions between all four systems and their components, these circuits should lend themselves to higher-level genetic circuitry for use in microbial consortia.
Keywords: crosstalk; microbial consortia; orthogonal; quorum sensing; synthetic ecology.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

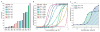
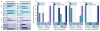
References
-
- Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. - PubMed
-
- McDaniel R, Weiss R. Advances in synthetic biology: on the path from prototypes to applications. Curr Opin Biotechnol. 2005;16:476–483. - PubMed
-
- Dueber JE, Wu GC, Malmirchegini GR, Moon TS, Petzold CJ, Ullal AV, Prather KL, Keasling JD. Synthetic protein scaffolds provide modular control over metabolic flux. Nat Biotechnol. 2009;27:753–759. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

