Identifying and targeting determinants of melanoma cellular invasion
- PMID: 27172792
- PMCID: PMC5173051
- DOI: 10.18632/oncotarget.9227
Identifying and targeting determinants of melanoma cellular invasion
Abstract
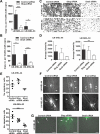
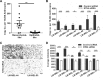
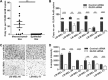
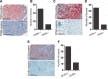
Epithelial-to-mesenchymal transition is a critical process that increases the malignant potential of melanoma by facilitating invasion and dissemination of tumor cells. This study identified genes involved in the regulation of cellular invasion and evaluated whether they can be targeted to inhibit melanoma invasion. We identified Peroxidasin (PXDN), Netrin 4 (NTN4) and GLIS Family Zinc Finger 3 (GLIS3) genes consistently elevated in invasive mesenchymal-like melanoma cells. These genes and proteins were highly expressed in metastatic melanoma tumors, and gene silencing led to reduced melanoma invasion in vitro. Furthermore, migration of PXDN, NTN4 or GLIS3 siRNA transfected melanoma cells was inhibited following transplantation into the embryonic chicken neural tube compared to control siRNA transfected melanoma cells. Our study suggests that PXDN, NTN4 and GLIS3 play a functional role in promoting melanoma cellular invasion, and therapeutic approaches directed toward inhibiting the action of these proteins may reduce the incidence or progression of metastasis in melanoma patients.
Keywords: embryonic chicken transplantation; epithelial-to-mesenchymal transition; invasion; melanoma.
Conflict of interest statement
None to declare.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

