Properties of acid-induced currents in mouse dorsal root ganglia neurons
- PMID: 27173673
- PMCID: PMC4873640
- DOI: 10.14814/phy2.12795
Properties of acid-induced currents in mouse dorsal root ganglia neurons
Abstract
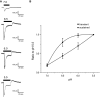
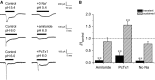
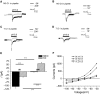
Acid-sensing ion channels (ASICs) are cation channels that are activated by protons (H(+)). They are expressed in neurons throughout the nervous system and may play important roles in several neurologic disorders including inflammation, cerebral ischemia, seizures, neurodegeneration, anxiety, depression, and migraine. ASICs generally produce transient currents that desensitize in response to a decrease in extracellular pH Under certain conditions, the inactivation of ASICs can be incomplete and allow them to produce sustained currents. Here, we characterize the properties of both transient and sustained acid-induced currents in cultured mouse dorsal root ganglia (DRG) neurons. At pH levels between 7.3 and 7.1 they include "window currents" through ASICs. With stronger acid signals sustained currents are maintained in the absence of extracellular Na(+) or the presence of the ASIC blockers amiloride and Psalmotoxin-1(PcTx1). These sustained responses may have several different origins in these cells, including acid-induced stimulation of inward Cl(-) currents, block of outward K(+) currents, and augmentation of inward H(+) currents, properties that distinguish these novel sustained currents from the well-characterized transient currents.
Keywords: ASIC; Amiloride; Pc1Tx; Zn2+; sustained currents.
© 2016 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures



Similar articles
-
Morphine inhibits acid-sensing ion channel currents in rat dorsal root ganglion neurons.Brain Res. 2014 Mar 20;1554:12-20. doi: 10.1016/j.brainres.2014.01.042. Epub 2014 Feb 1. Brain Res. 2014. PMID: 24491633
-
Endomorphins potentiate acid-sensing ion channel currents and enhance the lactic acid-mediated increase in arterial blood pressure: effects amplified in hindlimb ischaemia.J Physiol. 2017 Dec 1;595(23):7167-7183. doi: 10.1113/JP275058. Epub 2017 Nov 9. J Physiol. 2017. PMID: 29044528 Free PMC article.
-
Protons and Psalmotoxin-1 reveal nonproton ligand stimulatory sites in chicken acid-sensing ion channel: Implication for simultaneous modulation in ASICs.Channels (Austin). 2014;8(1):49-61. doi: 10.4161/chan.26978. Epub 2013 Nov 21. Channels (Austin). 2014. PMID: 24262969 Free PMC article.
-
A molecular view of the function and pharmacology of acid-sensing ion channels.Pharmacol Res. 2020 Apr;154:104166. doi: 10.1016/j.phrs.2019.02.005. Epub 2019 Feb 5. Pharmacol Res. 2020. PMID: 30731197 Review.
-
Research strategies for pain in lumbar radiculopathy focusing on acid-sensing ion channels and their toxins.Curr Top Med Chem. 2015;15(7):617-30. doi: 10.2174/1568026615666150217112652. Curr Top Med Chem. 2015. PMID: 25686734 Review.
References
-
- Adams, C. M. , Snyder P. M., and Welsh M. J.. 1999. Paradoxical stimulation of a DEG/ENaC channel by amiloride. J. Biol. Chem. 274:15500–15504. - PubMed
-
- Baron, A. , Schaefer L., Lingueglia E., Champigny G., and Lazdunski M.. 2001. Zn2 + and H+ are coactivators of acid‐sensing ion channels. J. Biol. Chem. 276:35361–35367. - PubMed
-
- Baron, A. , Diochot S., Salinas M., Deval E., Noel J., and Lingueglia E.. 2013. Venom toxins in the exploration of molecular, physiological and pathophysiological functions of acid‐sensing ion channels. Toxicon 75:187–204. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

