Suppression of Breast Cancer Cell Migration by Small Interfering RNA Delivered by Polyethylenimine-Functionalized Graphene Oxide
- PMID: 27173676
- PMCID: PMC4864886
- DOI: 10.1186/s11671-016-1463-0
Suppression of Breast Cancer Cell Migration by Small Interfering RNA Delivered by Polyethylenimine-Functionalized Graphene Oxide
Abstract
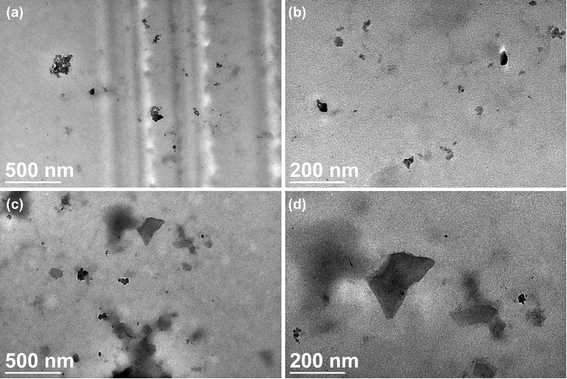
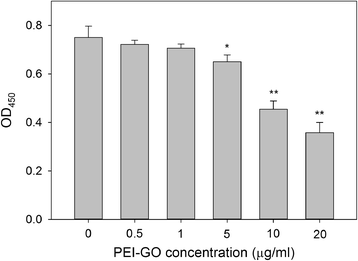
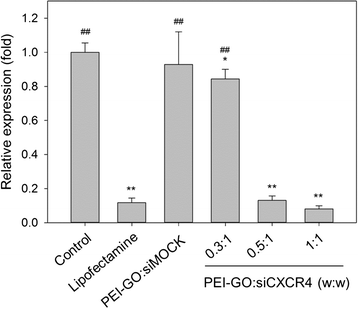
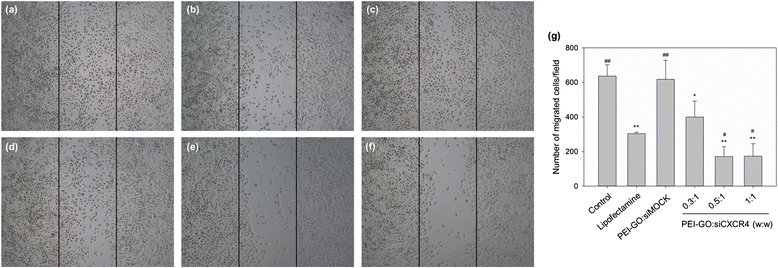
The carbon-based nanomaterial graphene can be chemically modified to associate with various molecules such as chemicals and biomolecules and developed as novel carriers for drug and gene delivery. In this study, a nonviral gene transfection reagent was produced by functionalizing graphene oxide (GO) with a polycationic polymer, polyethylenimine (PEI), to increase the biocompatibility of GO and to transfect small interfering RNA (siRNA) against C-X-C chemokine receptor type 4 (CXCR4), a biomarker associated with cancer metastasis, into invasive breast cancer cells. PEI-functionalized GO (PEI-GO) was a homogeneous aqueous solution that remained in suspension during storage at 4 °C for at least 6 months. The particle size of PEI-GO was 172 ± 4.58 and 188 ± 5.00 nm at 4 and 25 °C, respectively, and increased slightly to 262 ± 17.6 nm at 37 °C, but remained unaltered with time. Binding affinity of PEI-GO toward siRNA was assessed by electrophoretic mobility shift assay (EMSA), in which PEI-GO and siRNA were completely associated at a PEI-GO:siRNA weight ratio of 2:1 and above. The invasive breast cancer cell line, MDA-MB-231, was transfected with PEI-GO in complex with siRNAs against CXCR4 (siCXCR4). Suppression of the mRNA and protein expression of CXCR4 by the PEI-GO/siCXCR4 complex was confirmed by real-time PCR and western blot analysis. In addition, the metastatic potential of MDA-MB-231 cells was attenuated by the PEI-GO/siCXCR4 complex as demonstrated in wound healing assay. Our results suggest that PEI-GO is effective in the delivery of siRNA and may contribute to targeted gene therapy to suppress cancer metastasis.
Keywords: C-X-C chemokine receptor type 4 (CXCR4); Cancer cell migration; Graphene oxide (GO); Polyethylenimine (PEI); Small interfering RNA (siRNA).
Figures



Similar articles
-
Dataset on cytotoxicity effect of polyethylenimine-functionalized graphene oxide nanoparticles on the human embryonic carcinoma stem cell, NTERA2 cell line.Data Brief. 2019 Sep 6;26:104487. doi: 10.1016/j.dib.2019.104487. eCollection 2019 Oct. Data Brief. 2019. PMID: 31667251 Free PMC article.
-
Delivery of small interfering RNAs in human cervical cancer cells by polyethylenimine-functionalized carbon nanotubes.Nanoscale Res Lett. 2013 Jun 6;8(1):267. doi: 10.1186/1556-276X-8-267. Nanoscale Res Lett. 2013. PMID: 23742156 Free PMC article.
-
Combinational siRNA delivery using hyaluronic acid modified amphiphilic polyplexes against cell cycle and phosphatase proteins to inhibit growth and migration of triple-negative breast cancer cells.Acta Biomater. 2018 Jan 15;66:294-309. doi: 10.1016/j.actbio.2017.11.036. Epub 2017 Nov 26. Acta Biomater. 2018. PMID: 29183848
-
Functionalized Folate-Modified Graphene Oxide/PEI siRNA Nanocomplexes for Targeted Ovarian Cancer Gene Therapy.Nanoscale Res Lett. 2020 Mar 6;15(1):57. doi: 10.1186/s11671-020-3281-7. Nanoscale Res Lett. 2020. PMID: 32140846 Free PMC article.
-
Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo.Nanotechnology. 2013 Mar 15;24(10):105102. doi: 10.1088/0957-4484/24/10/105102. Epub 2013 Feb 20. Nanotechnology. 2013. PMID: 23425941
Cited by
-
Carbon-based Nanomaterials for Delivery of Small RNA Molecules: A Focus on Potential Cancer Treatment Applications.Pharm Nanotechnol. 2022;10(3):164-181. doi: 10.2174/2211738510666220606102906. Pharm Nanotechnol. 2022. PMID: 35670355 Review.
-
Graphene-based nanomaterials for breast cancer treatment: promising therapeutic strategies.J Nanobiotechnology. 2021 Jul 15;19(1):211. doi: 10.1186/s12951-021-00902-8. J Nanobiotechnology. 2021. PMID: 34266419 Free PMC article. Review.
-
Multifunctionalization of graphene and graphene oxide for controlled release and targeted delivery of anticancer drugs.Am J Transl Res. 2017 Dec 15;9(12):5197-5219. eCollection 2017. Am J Transl Res. 2017. PMID: 29312477 Free PMC article. Review.
-
Gene Therapy in Cancer Treatment: Why Go Nano?Pharmaceutics. 2020 Mar 5;12(3):233. doi: 10.3390/pharmaceutics12030233. Pharmaceutics. 2020. PMID: 32151052 Free PMC article. Review.
-
Magnetic chitosan/graphene oxide composite loaded with novel photosensitizer for enhanced photodynamic therapy.RSC Adv. 2018 Mar 14;8(19):10376-10388. doi: 10.1039/c8ra00747k. eCollection 2018 Mar 13. RSC Adv. 2018. PMID: 35540483 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

