Sipjeondaebo-tang in patients with cancer with anorexia: a protocol for a pilot, randomised, controlled trial
- PMID: 27173813
- PMCID: PMC4874172
- DOI: 10.1136/bmjopen-2016-011212
Sipjeondaebo-tang in patients with cancer with anorexia: a protocol for a pilot, randomised, controlled trial
Abstract
Introduction: Cancer-related anorexia is the loss of appetite or desire to eat in patients with cancer. Although treatments for cancer-related anorexia do exist, patients have sought complementary and alternative medicine including herbal remedies, due to safety concerns. Sipjeondaebo-tang is one among other popular herbal medicines that are beneficial to management of anorexia in Korea. The purpose of this study is to examine the feasibility for a full randomised clinical trial of Sipjeondaebo-tang for cancer-related anorexia.
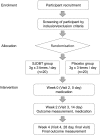
Methods and analysis: This study is a randomised, double-blinded and placebo-controlled trial of Sipjeondaebo-tang. For the study, 40 patients with cancer, aged 20-80 years, who reported anorexia, will be recruited. The participants will receive either 3 g of Sipjeondaebo-tang or a placebo, 3 times a day for 4 weeks. The primary end point is a change in the anorexia/cachexia subscale (A/CS) of Functional Assessment of Anorexia/Cachexia Therapy (FAACT). The secondary end points include changes in the visual analogue scale (VAS) of appetite, cortisol and ghrelin. The outcomes will be measured on every visit. Each participant will visit once a week during 4 weeks.
Ethics and dissemination: The present study has been approved by the Institutional Review Board of the Dunsan Korean Medicine Hospital of Daejeon University (reference DJDSKH-15-03-2 (V.2.0)). The results will be disseminated in a peer-reviewed journal and scientific conference.
Trial registration number: NCT02468141; Pre-results.
Keywords: COMPLEMENTARY MEDICINE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
Similar articles
-
Efficacy and Safety of Sipjeondaebo-Tang for Anorexia in Patients with Cancer: A Pilot, Randomized, Double-Blind, Placebo-Controlled Trial.Evid Based Complement Alternat Med. 2017;2017:8780325. doi: 10.1155/2017/8780325. Epub 2017 Dec 26. Evid Based Complement Alternat Med. 2017. PMID: 29441116 Free PMC article.
-
Sipjeondaebo-tang in patients with breast cancer with fatigue: a protocol for a pilot, randomised, double-blind, placebo-controlled, cross-over trial.BMJ Open. 2018 Jul 6;8(7):e021242. doi: 10.1136/bmjopen-2017-021242. BMJ Open. 2018. PMID: 29982213 Free PMC article.
-
Efficacy and safety of Yukgunja-Tang for treating anorexia in patients with cancer: The protocol for a pilot, randomized, controlled trial.Medicine (Baltimore). 2019 Oct;98(40):e16950. doi: 10.1097/MD.0000000000016950. Medicine (Baltimore). 2019. PMID: 31577697 Free PMC article.
-
Appetite problem in cancer patients: Pathophysiology, diagnosis, and treatment.Cancer Treat Res Commun. 2021;27:100336. doi: 10.1016/j.ctarc.2021.100336. Epub 2021 Feb 13. Cancer Treat Res Commun. 2021. PMID: 33607591 Review.
-
Ghrelin and its analogues as therapeutic agents for anorexia and cachexia in end-stage renal disease.Kidney Int. 2009 Jul;76(2):135-7. doi: 10.1038/ki.2009.74. Kidney Int. 2009. PMID: 19564855 Review.
Cited by
-
Current status of Complementary and Alternative Medicine Interventions in the Management of Pancreatic Cancer - An Overview.Curr Treat Options Oncol. 2023 Dec;24(12):1852-1869. doi: 10.1007/s11864-023-01146-4. Epub 2023 Dec 11. Curr Treat Options Oncol. 2023. PMID: 38079061 Free PMC article. Review.
-
Efficacy and Safety of Sipjeondaebo-Tang for Anorexia in Patients with Cancer: A Pilot, Randomized, Double-Blind, Placebo-Controlled Trial.Evid Based Complement Alternat Med. 2017;2017:8780325. doi: 10.1155/2017/8780325. Epub 2017 Dec 26. Evid Based Complement Alternat Med. 2017. PMID: 29441116 Free PMC article.
-
Effect of Sipjeondaebo-Tang on the Pharmacokinetics of S-1, an Anticancer Agent, in Rats Evaluated by Population Pharmacokinetic Modeling.Molecules. 2017 Sep 7;22(9):1488. doi: 10.3390/molecules22091488. Molecules. 2017. PMID: 28880240 Free PMC article.
-
The Role of Natural Products in the Improvement of Cancer-Associated Cachexia.Int J Mol Sci. 2023 May 15;24(10):8772. doi: 10.3390/ijms24108772. Int J Mol Sci. 2023. PMID: 37240117 Free PMC article. Review.
-
Sipjeondaebo-tang in patients with breast cancer with fatigue: a protocol for a pilot, randomised, double-blind, placebo-controlled, cross-over trial.BMJ Open. 2018 Jul 6;8(7):e021242. doi: 10.1136/bmjopen-2017-021242. BMJ Open. 2018. PMID: 29982213 Free PMC article.
References
-
- Dewys WD, Begg C, Lavin PT et al. . Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical