Symmetric spike timing-dependent plasticity at CA3-CA3 synapses optimizes storage and recall in autoassociative networks
- PMID: 27174042
- PMCID: PMC4869174
- DOI: 10.1038/ncomms11552
Symmetric spike timing-dependent plasticity at CA3-CA3 synapses optimizes storage and recall in autoassociative networks
Abstract
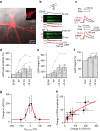
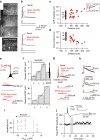
CA3-CA3 recurrent excitatory synapses are thought to play a key role in memory storage and pattern completion. Whether the plasticity properties of these synapses are consistent with their proposed network functions remains unclear. Here, we examine the properties of spike timing-dependent plasticity (STDP) at CA3-CA3 synapses. Low-frequency pairing of excitatory postsynaptic potentials (EPSPs) and action potentials (APs) induces long-term potentiation (LTP), independent of temporal order. The STDP curve is symmetric and broad (half-width ∼150 ms). Consistent with these STDP induction properties, AP-EPSP sequences lead to supralinear summation of spine [Ca(2+)] transients. Furthermore, afterdepolarizations (ADPs) following APs efficiently propagate into dendrites of CA3 pyramidal neurons, and EPSPs summate with dendritic ADPs. In autoassociative network models, storage and recall are more robust with symmetric than with asymmetric STDP rules. Thus, a specialized STDP induction rule allows reliable storage and recall of information in the hippocampal CA3 network.
Figures


References
-
- Kesner R. P. Behavioral functions of the CA3 subregion of the hippocampus. Learn. Mem. 14, 771–781 (2007). - PubMed
-
- Marr D. Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 262, 23–81 (1971). - PubMed
-
- McNaughton B. L. & Morris R. G. M. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 10, 408–415 (1987).
-
- Lisman J. E. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron 22, 233–242 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

