Mitochondrial Metabolism in Aging Heart
- PMID: 27174952
- PMCID: PMC5009371
- DOI: 10.1161/CIRCRESAHA.116.307505
Mitochondrial Metabolism in Aging Heart
Abstract
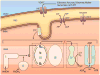
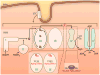
Altered mitochondrial metabolism is the underlying basis for the increased sensitivity in the aged heart to stress. The aged heart exhibits impaired metabolic flexibility, with a decreased capacity to oxidize fatty acids and enhanced dependence on glucose metabolism. Aging impairs mitochondrial oxidative phosphorylation, with a greater role played by the mitochondria located between the myofibrils, the interfibrillar mitochondria. With aging, there is a decrease in activity of complexes III and IV, which account for the decrease in respiration. Furthermore, aging decreases mitochondrial content among the myofibrils. The end result is that in the interfibrillar area, there is ≈50% decrease in mitochondrial function, affecting all substrates. The defective mitochondria persist in the aged heart, leading to enhanced oxidant production and oxidative injury and the activation of oxidant signaling for cell death. Aging defects in mitochondria represent new therapeutic targets, whether by manipulation of the mitochondrial proteome, modulation of electron transport, activation of biogenesis or mitophagy, or the regulation of mitochondrial fission and fusion. These mechanisms provide new ways to attenuate cardiac disease in elders by preemptive treatment of age-related defects, in contrast to the treatment of disease-induced dysfunction.
Keywords: cardiolipin; electron transport chain complex proteins; fatty acid oxidation complex; oxidative phosphorylation; reactive oxygen species.
© 2016 American Heart Association, Inc.
Conflict of interest statement
The authors state they have no conflict of interest or financial interests to disclose.
Figures




References
-
- Koonen DP, Febbraio M, Bonnet S, Nagendran J, Young ME, Michelakis ED, Dyck JR. Cd36 expression contributes to age-induced cardiomyopathy in mice. Circulation. 2007;116:2139–2147. - PubMed
-
- Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: Ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. - PubMed
-
- Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. - PubMed
-
- Calvani M, Reda E, Arrigoni-Martelli E. Regulation by carnitine of myocardial fatty acid and carbohydrate metabolism under normal and pathological conditions. Basic Res Cardiol. 2000;95:75–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

