Hydrogen bonding and anticancer properties of water-soluble chiral p-cymene Ru(II) compounds with amino-oxime ligands
- PMID: 27175101
- PMCID: PMC4862618
- DOI: 10.1002/ejic.201500097
Hydrogen bonding and anticancer properties of water-soluble chiral p-cymene Ru(II) compounds with amino-oxime ligands
Abstract
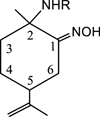
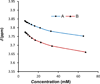
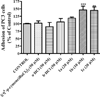
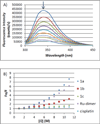
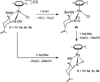
The investigation of the hydrogen-bonding effect on the aggregation tendency of ruthenium compounds [(η6-p-cymene)Ru(κNHR,κNOH)Cl]Cl (R = Ph (1a), Bn (1b)) and [(η6-p-cymene)Ru(κ2NH(2-pic),κNOH)][PF6]2 (1c), [(η6-p-cymene)Ru(κNHBn,κNO)Cl] (2b) and [(η6-p-cymene)Ru(κNBn,κ2NO)] (3b), has been performed by means of concentration dependence 1H NMR chemical shifts and DOSY experiments. The synthesis and full characterization of new compounds 1c, [(η6-p-cymene)Ru(κNPh,κ2NO)] (3a) and 3b are also reported. The effect of the water soluble ruthenium complexes 1a-1c on cytotoxicity, cell adhesion and cell migration of the androgen-independent prostate cancer PC3 cells have been assessed by MTT, adhesion to type-I-collagen and recovery of monolayer wounds assays, respectively. Interactions of 1a-1c with DNA and human serum albumin have also been studied. Altogether, the properties reported herein suggest that ruthenium compounds 1a-1c have considerable potential as anticancer agents against advanced prostate cancer.
Keywords: anticancer; arene ruthenium compounds; chiral syntheses; non-covalent interactions; oxime.
Figures





References
-
- Keppler BK. Metal Complexes in Cancer Chemotherapy. Weinheim: Wiley VCH; 1993.
- Barnes KR, Lippard SJ. In: Metal Ions in Biological Systems. Sigel A, Sigel H, editors. Vol. 42. Marcel Dekker Inc; 2004. pp. 179–208.
- van Rijt SH, Sadler PJ. Drug Discov. Today. 2009;14:1089–1097. - PMC - PubMed
- Gasser G, Ott I, Metzler-Nolte N. J. Med. Chem. 2011;54:3–25. - PMC - PubMed
- Hartinger CG, Metzler-Nolte N, Dyson PJ. Organometallics. 2012;31:5677–5685.
-
- Sava G, Bergamo A, Dyson PJ. Dalton Trans. 2011;40:9069–9075. - PubMed
-
- Melchart M, Sadler PJ. In: Bioorganometallics. Jaouen G, editor. Weinheim: Wiley VCH Verlag GmbH & Co. KGaA; 2006. pp. 39–64.
- Bergamo A, Sava G. Dalton Trans. 2011;40:7817–7823. - PubMed
- Smith GS, Therrien B. Dalton Trans. 2011;40:10793–10800. - PubMed
- Clavel CM, Paunescu E, Nowak-Sliwinska P, Griffioen AW, Scopelliti R, Dyson PJ. J. Med. Chem. 2014;57:3546–3558. - PubMed
- Nazarov AA, Hartinger CG, Dyson PJ. Journal of Organometallic Chemistry. 2014;751:251–260.
- Pettinari R, Marchetti F, Condello F, Pettinari C, Lupidi G, Scopelliti R, Mukhopadhyay S, Riedel T, Dyson PJ. Organometallics. 2014;33:3709–3715.
-
- Ang WH, Casini A, Sava G, Dyson PJ. J. Organomet. Chem. 2011;696:989–998.
-
- Bergamo A, Gaiddon C, Schellens JHM, Beijnen JH, Sava G. J. Inorg. Biochem. 2012;106:90–99. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
