The feasibility of progressive resistance training in women with polycystic ovary syndrome: a pilot randomized controlled trial
- PMID: 27175282
- PMCID: PMC4865007
- DOI: 10.1186/s13102-016-0039-8
The feasibility of progressive resistance training in women with polycystic ovary syndrome: a pilot randomized controlled trial
Abstract
Background: To evaluate the feasibility of executing a randomized controlled trial of progressive resistance training (PRT) in women with polycystic ovary syndrome (PCOS).
Methods: Women with PCOS were randomized to an experimental (PRT) group or a no-exercise (usual care) control group. The PRT group was prescribed two supervised and two unsupervised (home-based) training sessions per week for 12 weeks. Feasibility outcomes included recruitment and attrition, adherence, adverse events, and completion of assessments. Secondary outcomes, collected pre and post intervention, included a range of pertinent physiological, functional and psychological measures.
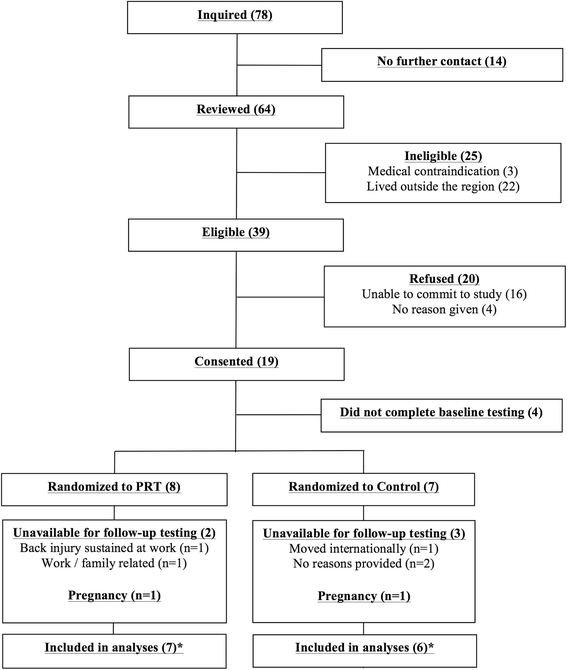
Results: Fifteen participants were randomised into the PRT group (n = 8) or control group (n = 7); five women (n = 2 in PRT group and n = 3 in control group) withdrew from the study. The most successful recruitment sources were Facebook (40 %) and online advertisement (27 %), while least successful methods were referrals by clinicians, colleagues and flyers. In the PRT group, attendance to supervised sessions was higher (95 %; standard deviation ±6 %) compared to unsupervised sessions (51 %; standard deviation ±28 %). No adverse events were attributed to PRT. Change in menstrual cycle status was not significantly different between groups over time (p = 0.503). However, the PRT group significantly increased body weight (p = 0.01), BMI (p = 0.04), lean mass (p = 0.01), fat-free mass (p = 0.005) and lower body strength (p = 0.03), while reducing waist circumference (p = 0.03) and HbA1c (p = 0.033) versus the control group. The PRT group also significantly improved across several domains of disease-specific and general health-related quality of life, depression, anxiety and exercise self-efficacy.
Conclusion: A randomized controlled trial of PRT in PCOS would be feasible, and this mode of exercise may elicit a therapeutic effect on clinically important outcomes in this cohort. The success of a large-scale trial required to confirm these findings would be contingent on addressing the feasibility hurdles identified in this study with respect to recruitment, attrition, compliance, and collection of standardized clinical data.
Trial registration: Australia New Zealand Clinical Trials Registry; ACTRN12614000517673 Registered 15 May 2014.
Keywords: Exercise; Menstrual cyclicity; Psychological health; Quality of life; Weight training.
Figures
References
-
- Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Work-shop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. - PubMed
-
- Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. 2007. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical