Relationship between clinical and patient-reported outcomes in a phase 3 trial of tofacitinib or MTX in MTX-naïve patients with rheumatoid arthritis
- PMID: 27175296
- PMCID: PMC4860866
- DOI: 10.1136/rmdopen-2015-000232
Relationship between clinical and patient-reported outcomes in a phase 3 trial of tofacitinib or MTX in MTX-naïve patients with rheumatoid arthritis
Abstract
Objective: To compare the relationship between clinical measures and patient-reported outcomes (PROs) in patients with rheumatoid arthritis (RA) treated with tofacitinib or methotrexate (MTX).
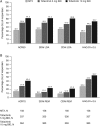
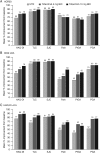
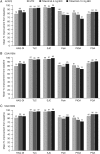
Methods: In a phase 3 randomised controlled trial, patients (N=956) who were MTX-naïve or had received ≤3 doses were randomised and received tofacitinib 5 or 10 mg twice daily or MTX titrated to 20 mg/week. Outcomes included: per cent of patients achieving American College of Rheumatology 70% responses (ACR70), ACR50, low disease activity (LDA) by Simplified Disease Activity Index (SDAI ≤11) and Clinical Disease Activity Index (CDAI ≤10), remission by SDAI (≤3.3) and CDAI (≤2.8), patient-reported Health Assessment Questionnaire-Disability Index (HAQ-DI scores <0.5), pain and global assessment of disease activity.
Results: At month 6, most patients who achieved LDA/remission by one definition achieved LDA/remission with others; however, discordance between measures was greater with MTX than with tofacitinib. As expected, concordance between CDAI and SDAI responses was high. Overall, patients achieving LDA or ACR50 responses reported less improvement in PROs (HAQ-DI, pain and patient global assessment) compared with clinical measures (tender and swollen joint counts).
Conclusions: Variability in levels of responses between clinical outcomes and PROs should be considered when setting treat-to-target goals in patients with RA.
Trial registration number: NCT01039688; Post-results.
Keywords: DMARDs (synthetic); Methotrexate; Patient perspective; Rheumatoid Arthritis.
Figures

References
-
- Pincus T, Richardson B, Strand V et al. . Relative efficiencies of the 7 rheumatoid arthritis Core Data Set measures to distinguish active from control treatments in 9 comparisons from clinical trials of 5 agents. Clin Exp Rheumatol 2014;32(Suppl 85): S47–54.10.1016/j.berh.2007.02.004 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
